Abstract
Purpose
This study aimed to compare the efficacy of human chorionic gonadotropin (hCG) and dual-trigger (gonadotropin-releasing hormone agonist [GnRH-a] and hCG) protocols in terms of cumulative live birth rate (CLBR) and other pregnancy outcomes among advanced-age women.
Methods
We enrolled 801 women aged ≥ 35 years who were experiencing infertility and beginning their first in vitro fertilization cycle at a tertiary academic medical institution between August 2015 and June 2023. Among these, 115 and 686 women used the dual-trigger and hCG methods. Propensity score matching was employed to account for confounding variables. The main outcomes evaluated were CLBR and time to live birth (TTLB).
Results
The CLBR did not differ significantly between the hCG and dual-trigger groups (29.86% vs. 26.09%, P = 0.44), whereas the TTLBs of both groups were similar (9.60 vs. 10.14 months, P = 0.72). CLBR results were similar for both groups, according to a Kaplan–Meier analysis (hazard ratio [HR] = 0.95; 95% confidence interval [CI] 0.63–1.43; P = 0.82). After a multiple Cox proportional hazards model was established, the CLBR of the hCG group remained comparable with that of the dual-trigger group (HR = 0.83; 95% CI 0.53–4.11; P = 0.39). The subgroup analysis also showed similar findings.
Conclusion
Considering the higher fertilization rate and shorter TTLB, the dual-trigger protocol may be more suitable than the hCG trigger protocol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This study compared the efficacy of hCG and dual-trigger protocols in advanced-age women undergoing in vitro fertilization based on propensity score matching, Kaplan–Meier curves, and Cox proportional hazards models. |
Introduction
The age of conception has a significant impact on human reproductive success and is steadily increasing globally [1]. Age-related reductions in follicle quality and quantity continue to affect in vitro fertilization (IVF) pregnancy rates and fertility [2,3,4]. Studies show that women aged > 35 years experience a significant decline in ovarian reserve and weakened responsiveness to ovulation induction, resulting in comparatively poorer outcomes in assisted reproductive technology pregnancy [5, 6]. Advanced-age women are more prone to embryo implantation failure and miscarriage, further reducing the likelihood of a successful pregnancy [7].
During IVF, the ovulation trigger protocol is essential for maintaining luteal function and stabilizing hormone levels. Gonadotropins stimulate oocyte growth, and their final maturation is usually triggered by human chorionic gonadotropin (hCG) [8, 9]. However, the long-lasting luteotropic effect of hCG is one significant risk element contributing to the onset of ovarian hyperstimulation syndrome (OHSS) [10]. hCG also lacks follicle-stimulating hormone (FSH) activity, which is necessary for oocyte maturation in vitro [11]. The use of a gonadotropin-releasing hormone agonist (GnRH-a) as a single-trigger agent simulates LH surge in the natural cycle, benefits oocyte maturation, and lowers OHSS risk [12, 13]. However, the pregnancy rate and live birth rate (LBR) are significantly reduced when triggered by GnRH-a alone [14].
The dual-trigger protocol, which combines hCG and GnRH-a, is being applied in many clinical settings due to its potential to enhance various outcomes, including but not limited to oocyte maturation rates [15], number of good-quality embryos [16], and LBR [17], while posing a minimal risk of OHSS [18]. However, studies on trigger methods for IVF outcomes typically include heterogeneous populations with inconsistent results, highlighting the need for further high-quality research. Therefore, this study aimed to assess the effectiveness of both trigger methods regarding cumulative LBR (CLBR) and various other pregnancy outcomes among advanced-age women throughout a single IVF cycle. This cycle comprised fresh embryo transfers along with any subsequent frozen embryo transfers (FET).
Materials and methods
Ethics approval and informed consent
The Ethics Committee of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine evaluated and approved this study protocol and waived the requirement for informed consent due to the study’s retrospective and anonymous design. This study was conducted following the principles of the Declaration of Helsinki and its amendments [19].
Study design and population
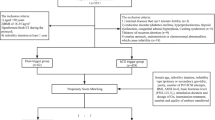
Women of advanced maternal age (AMA) (≥ 35 years) who had their first IVF cycle and embryo transfer procedure at a tertiary academic medical facility between August 2015 and June 2023 were included in this study. Each participant was provided an ovarian stimulation protocol that included a gonadotropin-releasing hormone antagonist (GnRH-ant). Women were excluded from the study if they (1) had undergone ovarian surgery, had a history of genital tumors, experienced endometriosis, or had uterine malformations; (2) were involved in cycles utilizing cryopreserved oocytes and/or donor oocytes; (3) participated in cycles that involved pre-implantation genetic testing or diagnosis; or (4) experienced recurrent spontaneous abortions (≥ 3 times). Ultimately, 801 patients were enrolled, and they were assigned to the dual (co-administration of GnRH-a and hCG) and hCG trigger groups.
Ovarian stimulation protocol
On the second or third day of the menstrual cycle, 100–300 IU/d of recombinant human FSH (r-FSH) (Gonal-F, Merck Serono, Switzerland) were administered, depending on the body mass index (BMI), antral follicle count (AFC), maternal age, and basic ovarian endocrine test. Blood hormone assays and serial transvaginal ultrasound were used to track the participants’ follicular recruitment, growth, and endometrial thickness during controlled ovarian stimulation (COS). Within the 50-IU range, the r-FSH dosage can be raised or lowered based on the patient’s follicular growth during COS. When the dominant follicle diameter was 12–14 mm and E2 levels were ≥ 400 pg/mL, a daily dose of 0.25 mg of GnRH-ant (cetrorelix acetate, Cetrotide; Merck Serono, Germany) was administered until the trigger day. GnRH-a (triptorelin acetate, Ipsen Pharma [Biotech], France) in combination with hCG (dual trigger) or hCG (Lizhu, Zhuhai, China) was used to initiate when two or more follicles were at least 18 mm in size. Women who were in the dual-trigger group were administered 0.2 mg of GnRH-a subcutaneously and 2000–4000 IU of hCG intramuscularly, and those in the hCG group received 4000–10,000 IU of hCG intramuscularly. The trigger method was based on the doctors’ preferences, and oocyte retrieval was performed approximately 35–37 h later.
IVF and embryo evaluation
IVF was used to inseminate the retrieved oocytes unless intracytoplasmic sperm injection (ICSI) was recommended due to a total count of progressively motile sperm being below 5 × 106 or when the sperm exhibited normal morphology in < 1% of the samples. Cleavage-stage embryos and blastocysts were graded per the Istanbul consensus [20] and Gardner grading systems [21]. High-quality embryos were characterized as blastocysts graded 3BB or higher, cleavage-stage embryos with at least six blastomeres, and 20% fragmentation.
Embryo transfer and luteal support
One or two embryos can be transferred on day 3 (cleavage embryo) or 5 (blastocyst) following oocyte retrieval. A previously established quick-freezing procedure was used to cryopreserve extra embryos or high-quality blastocysts [22]. The freeze-all strategy was implemented when P on the trigger day was ≥ 2 ng/mL, endometrial thickness was ≤ 6 mm or ≥ 18 mm, or when endometrial polyps, intrauterine fluid, or a tendency toward ovarian hyperstimulation were observed. Ovulatory women were administered the FET regimen throughout the natural or modified cycles, whereas women with anovulation or ovulatory disorders received ovulation induction treatment or hormonal replacement cycles. For luteal phase support (LPS) up to 10–12 weeks of gestation, oral P in combination with vaginal or intramuscular P was administered to all patients undergoing embryo transfer.
Outcome measurements
We compared IVF and pregnancy outcomes with different trigger methods. The primary outcome was the CLBR for each IVF cycle. A live birth was characterized as any instance of birth in which at least one infant, born alive and gestated for over 28 weeks, was delivered. CLBR was measured as the total count of the first live birth cycles following the transfer of all embryos, including fresh and frozen-thawed ones, produced during IVF treatments. The co-primary outcome was the number of months from oocyte retrieval to delivery, defined as time to live birth (TTLB). Secondary outcomes included the rates of implantation, clinical pregnancy, early pregnancy loss, ongoing pregnancy, live birth, and IVF results. Implantation is the process in which a fertilized egg is attached to the uterine wall. Clinical pregnancy was confirmed when at least one gestational sac was detected during a transvaginal ultrasound examination. An ectopic pregnancy is a pregnancy that arises outside of the uterine cavity. Early pregnancy loss was characterized as the spontaneous end of a clinically acknowledged pregnancy before reaching 12 weeks of gestation. Ongoing pregnancy was identified as an intrauterine pregnancy that continued for a minimum of 20 weeks.
Statistical analysis
According to the sample size calculation method for the Cox proportional hazards model [23], the R package powerSurvEpi was used for the computation. Based on the specified parameters: significance level (α) = 0.05, statistical power (1−β) = 80%, hazard ratio (HR) = 0.8, and group allocation ratio = 1:3 (dual trigger: hCG trigger), the minimum required sample size was determined to be 430 participants (108 in the dual trigger group and 322 in the hCG trigger group). Standard propensity score matching (PSM) was performed after multiple imputations for missing values due to the large sample size difference between the groups. Nearest-neighbor matching was used with a caliper of 0.02. All baseline characteristics were matched in a ratio of 1:3 without replacement. Q-Q plots, Shapiro–Wilk tests, and histograms were used to test for normality. Continuous variables were presented as mean ± standard deviation or median [25th, 75th], whereas categorical variables were expressed as frequency (n) and percentage (%). When necessary, the chi-square test, Mann–Whitney U test, or student’s t-test were used to evaluate differences. The log-rank test facilitated the comparison of cumulative live birth events between the hCG and dual-trigger protocol groups using Kaplan–Meier curves. To assess the influence of trigger patterns, both univariate and multivariate Cox proportional hazard models were used to compute the hazard ratios (HRs) along with 95% confidence intervals (CIs) for CLBR. Sensitivity analysis was conducted on the subpopulations undergoing fresh embryo transfer and those participating in freeze-all cycles. All statistical analyses were performed using R software version 4.3.1 (R Core Team, Vienna, Austria) and IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, N.Y., USA). Statistical significance was considered at P < 0.05.
Results
A total of 981 women were evaluated for eligibility, among whom 801 were included in this study (686 and 115 using the hCG and dual-trigger protocols, respectively). This study comprised 133 women who had at least one live birth (Fig. 1).
Baseline characteristics before and after matching
Table 1 shows the baseline characteristics before PSM, with no significant differences except for basal endometrial thickness (P < 0.05). The standardized mean differences (SMD) in AFC, basal FSH and P levels, infertility factors, basal endometrial thickness, and basal E2 level exceeded 0.1. To minimize bias, cycles were matched 1:3 using 11 variables, yielding a comparable control group (115 vs. 345 women). Table 2 shows post-matching characteristics, with no significant differences (P > 0.05) and all SMDs within 0.1. The SMD before and after PSM is shown in Supplementary Fig. 1.
Ovarian stimulation outcomes
Table 3 presents the outcomes of ovarian stimulation. Regarding the duration of stimulation, women undergoing the hCG protocol experienced a shorter time than those undergoing the dual-trigger protocol (P = 0.04). The dual-trigger group experienced cetrorelix for a shorter time and used significantly less cetrorelix than the hCG group (P < 0.01 and P < 0.001). Fresh embryo transfers were more frequent in the hCG group than in the dual-trigger group (54.49% vs. 40.0%), while the freeze-all method was more common in the dual-trigger group (47.83% vs. 33.91%). In addition to the above four aspects, both groups demonstrated comparable outcomes.
Embryological and pregnancy outcomes
Table 4 presents the embryological outcomes of the two groups. The hCG trigger group underwent more fresh ET cycles, while the dual-trigger group underwent more FET cycles (P < 0.02). IVF fertilization rate was comparable between both groups, whereas the ICSI fertilization rate was higher in women in the dual-trigger group (74.09% vs. 85.28%, P < 0.01). No significant differences were identified in the remaining embryological outcomes between the two groups (P > 0.05). For fresh transfer cycles and FET cycles, the implantation rate, clinical pregnancy rate, ectopic pregnancy rate, early pregnancy loss rate, ongoing pregnancy rate, and LBR showed no notable variations between both groups (P > 0.05) (Table 5). Similarly, subgroup analyses based on ovarian reserve markers, including AMH and AFC, revealed no statistically significant differences between the dual-trigger and the hCG trigger group in any stratum (all P > 0.05) (Supplementary Table 1 and Supplementary Table 2).
CLBR and TTLB
The CLBR (P = 0.44) and TTLB (P = 0.72, Table 5) were similar in the hCG and dual-trigger groups. Figure 2 shows the Kaplan–Meier results for CLBR. The median TTLB in the entire population was 9.6 and 10.1 months. Additionally, the log-rank test found no significant change in CLBR (P = 0.82, HR = 0.95; 95% CI 0.63–1.43, Fig. 2a). After stratifying by embryo transfer protocol, the results did not differ between women with fresh embryo transfer (P = 0.11, HR = 0.62; 95% CI 0.34–1.11, Fig. 2b) and those with freeze-all cycles (P = 0.59, HR = 0.85; 95% CI 0.47–1.54, Fig. 2c). We also used Cox proportional hazards models to assess the impact of hCG or dual-trigger strategy on CLBR (Table 6). In the adjusted model, controlling for age, BMI, type and years of infertility, infertility factors; basal FSH, LH, E2, and P levels; basal endometrial thickness, AFC, and embryo stage at transfer, women undergoing the hCG protocol showed a CLBR similar to that of those undergoing the dual-trigger protocol (HR = 0.83; 95% CI 0.53–4.11; P = 0.39). For sensitivity analysis, Cox models were run in subgroups based on embryo transfer strategy (Table 6). Women who had fresh embryo transfer (HR = 0.61; 95% CI 0.30–1.22; P = 0.16) or freeze-all embryos (HR = 0.75; 95% CI 0.35–1.59; P = 0.54) showed comparable CLBR irrespective of whether they followed the hCG or dual-trigger strategy.
Discussion
hCG and GnRH-a have drawbacks as triggering agents, and hCG increases the risk of OHSS [10]. However, after releasing a large amount of endogenous LH and FSH, the secretion of LH and P is suppressed [24], leading to impaired pregnancy outcomes [25]. Patients with poor response to GnRH-a still encounter low oocyte retrieval rates [26]. Although combining hCG and GnRH-a can mitigate the adverse effects of the two triggering protocols, the dose is reduced, weakening its sustained luteotropic effect. Many studies have compared the beneficial effects of dual-trigger and hCG-alone protocols. However, research on these methods, specifically in women with AMA, is relatively limited.
This study revealed a small but not statistically significant increase in the number of retrieved and mature oocytes in women receiving dual triggers compared to those receiving hCG alone, aligning with previously published prospective randomized controlled trials (RCTs) [16]. Similar findings were also reported in several retrospective studies [15, 27]. In studies focusing on different ovarian responders, the GnRH-a trigger group consistently exhibited a larger proportion of metaphase II (MII) oocytes than the hCG group [17, 28, 29]. One advantage of GnRH-a triggering is that it induces a mid-cycle FSH surge, mimicking hormonal changes in the natural ovulation cycle. Animal studies have demonstrated that FSH increases LH receptor sites in granulosa cells [30], and LH receptor expression is essential for the LH surge and luteinization of granulosa cells in pre-ovulatory mature follicles. FSH is crucial for promoting oocyte meiotic resumption [31, 32] and cumulus cell expansion [33, 34]. Thus, GnRH-a improves the retrieval rate of mature oocytes. A study by Griffin et al. revealed that patients with low-maturity oocytes exhibited significantly improved oocyte maturity in subsequent IVF cycles with dual triggering using standard hCG and GnRH-a doses [15]. Two RCTs demonstrated improved final follicular maturation following dual triggering [35, 36]. However, in this study, the MII oocyte rate in the dual-trigger group was slightly lower, but not statistically different owing to our inclusion of women with AMA with a median age of 38 years in both groups, considered a turning point for significant fertility decline [37]. Ovarian stimulation duration, antagonist duration, and total antagonist use also significantly differed in this study. In the dual-trigger group, extended ovarian stimulation could cause follicular overmaturity and aging, therefore limited GnRH-a use may not sufficiently suppress the LH surge, possibly contributing to bias in our results.
However, the significantly higher ICSI oocyte fertilization rate observed in this study suggests enhanced oocyte competence with dual triggering. After comparing the differential expression of reproductive-related gene messenger RNA in the granulosa cells of oocytes from both groups, Haas et al. found that the dual-trigger group expressed higher levels of amphiregulin and epiregulin [38], which are epidermal growth factor receptor ligands, and both participate in cumulus expansion, oocyte maturation, and meiotic resumption [39, 40]. The number of 2PN embryos and high-quality embryo rate were approximately equal, indicating no direct improvement in embryonic development with dual triggering. However, previous studies indicated that older women with dual-trigger protocol had considerably more high-quality and viable embryos than those in the single-trigger group [16, 36, 41]. The differences in the study results may stem from variations in the population and sample size.
Although the dual-trigger group demonstrated a higher ICSI oocyte fertilization rate, this did not translate to improved clinical outcomes. This discrepancy may be attributed to factors such as embryo quality, endometrial receptivity [42], and baseline patient characteristics (e.g., age) [43], highlighting the complexity of reproductive outcomes beyond fertilization success. The dual-trigger group had numerically lower implantation and clinical pregnancy rates after fresh ET than the hCG group, whereas these rates were numerically higher in the FET cycles, although none of these differences reached statistical significance. Previous studies revealed that pregnancy outcomes of FET are more strongly related to embryo quality [44] and that dual triggering improves oocyte maturation and embryo development in older women [16]. The poorer outcomes of fresh embryo transfer cycles in the dual-trigger group may be due to GnRH-a’s negative impact on luteal function. Its shorter half-life induces early luteolysis, reducing steroidal and non-steroidal hormones and impairing endometrial receptivity [45, 46].
The subgroup analyses for AMH and AFC were stratified according to the ESHRE definition of poor ovarian response (POR) [47]. No significant differences in pregnancy outcomes were observed between the two groups. This may be attributed to the relatively small sample size. A previous study on normal ovarian responders (NOR) have also reported no significant differences in outcomes between the dual trigger and the hCG group, except for CLBR [48].
However, the study had certain drawbacks. First, the study’s retrospective, single-center design and small sample size call into question the credibility of its conclusions. Therefore, randomized, multicenter, double-blind, placebo-controlled trials are required. Additionally, developing a clinical prediction model could help facilitate personalized interventions and improve clinical pregnancy outcomes in assisted reproductive technology [49]. Another limitation involves potential selection bias in trigger protocol assignment. In our study, physician discretion, patient characteristics, and the perceived risk of OHSS played a key role in determining whether to use an hCG-only trigger or a dual trigger. Specifically, patients with a higher AFC or elevated estradiol levels were more likely to receive the dual trigger to mitigate OHSS risk, whereas those with lower ovarian response tended to receive the hCG trigger. Additionally, women with AMA constitute a fairly heterogeneous population, with significant variability in ovarian reserve function, response to ovarian stimulation, and underlying fertility factors. This heterogeneity is further complicated by the overlap between patients with POR, diminished ovarian reserve, and NOR. Future studies could achieve more precise results by grouping participants based on additional stratification factors, such as FSH levels and basal endometrial thickness.
Conclusion
In our study, various trigger protocols did not affect long-term reproductive outcomes in women with AMA. However, when compared to a single hCG trigger, dual treatment improves fertilization rates in women with AMA. Considering the higher fertilization rate, the dual-trigger protocol may be more suitable than the hCG trigger protocol. In addition, Long-term multicenter prospective studies with larger sample sizes are needed to validate our findings.
Data availability
No datasets were generated or analysed during the current study.
References
Schmidt L, Sobotka T, Bentzen JG, Nyboe Andersen A, on behalf of the ER, Society Task F (2012) Demographic and medical consequences of the postponement of parenthood. Hum Reprod Update 18:29–43. https://doi.org/10.1093/humupd/dmr040
Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi N, Ubaldi FM, Rienzi L (2018) Impact of maternal age on oocyte and embryo competence. Front Endocrinol (Lausanne) 9:327. https://doi.org/10.3389/fendo.2018.00327
Eichenlaub-Ritter U (2012) Oocyte ageing and its cellular basis. Int J Dev Biol 56:841–852. https://doi.org/10.1387/ijdb.120141ue
Wang ZY, Huang SX, Yang JD, Li DP, Xu YW (2023) Subfertile Chinese patients with diminished ovarian reserve: an analysis of pregnancy outcomes of ART cycles. Pak J Med Sci 39:338–343. https://doi.org/10.12669/pjms.39.2.6226
Kesharwani DK, Mohammad S, Acharya N, Joshi KS (2022) Fertility with early reduction of ovarian reserve. Cureus 14:e30326. https://doi.org/10.7759/cureus.30326
Shrem G, Salmon-Divon M, Mahfoudh AM, Balayla J, Volodarsky-Perel A, Henderson S, Zeadna A, Son W-Y, Steiner N, Dahan MH (2022) Influence of maternal age and ovarian reserve on the decision to continue or to cancel IVF cycles in patients with one or two large follicles: a dual effect. Reprod Sci 29:291–300. https://doi.org/10.1007/s43032-021-00649-5
Zhang W, Zhang L, Liu Y, Li J, Xu X, Niu W, Xu J, Sun B, Guo Y (2021) Higher chromosomal aberration frequency in products of conception from women older than 32 years old with diminished ovarian reserve undergoing IVF/ICSI. Aging (Albany NY) 13:10128–10140. https://doi.org/10.18632/aging.202772
Kol S, Humaidan P (2010) LH (as HCG) and FSH surges for final oocyte maturation: sometimes it takes two to tango? Reprod Biomed Online 21:590–592. https://doi.org/10.1016/j.rbmo.2010.06.031
LudwigDoody MKJ, Doody KM (2003) Use of recombinant human chorionic gonadotropin in ovulation induction. Fertil Steril 79:1051–1059. https://doi.org/10.1016/S0015-0282(03)00173-0
Delvigne A, Rozenberg S (2002) Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update 8:559–577. https://doi.org/10.1093/humupd/8.6.559
Pandey S, Vaishnav P, Yadav A (2023) #210: the comparison of agonist trigger and dual trigger in our IVF population-a single center retrospective study. Fertil Reprod 05:365–367. https://doi.org/10.1142/S2661318223741693
Humaidan P, Bredkjaer HE, Bungum L, Bungum M, Grøndahl ML, Westergaard L, Andersen CY (2005) GnRH agonist (buserelin) or hCG for ovulation induction in GnRH antagonist IVF/ICSI cycles: a prospective randomized study. Hum Reprod 20:1213–1220. https://doi.org/10.1093/humrep/deh765
Shapiro BS, Andersen CY (2015) Major drawbacks and additional benefits of agonist trigger–not ovarian hyperstimulation syndrome related. Fertil Steril 103:874–878. https://doi.org/10.1016/j.fertnstert.2015.01.035
Shapiro M, Romanski P, Thomas A, Lanes A, Yanushpolsky E (2021) Low dose hCG supplementation in a Gn-RH-agonist trigger protocol is associated with worse pregnancy outcomes: a retrospective cohort study. Fertil Res Pract 7:12. https://doi.org/10.1186/s40738-021-00104-8
Griffin D, Feinn R, Engmann L, Nulsen J, Budinetz T, Benadiva C (2014) Dual trigger with gonadotropin-releasing hormone agonist and standard dose human chorionic gonadotropin to improve oocyte maturity rates. Fertil Steril 102:405–409. https://doi.org/10.1016/j.fertnstert.2014.04.028
Zhou C, Yang X, Wang Y, Xi J, Pan H, Wang M, Zhou Y, Xiao Y (2022) Ovulation triggering with hCG alone, GnRH agonist alone or in combination? A randomized controlled trial in advanced-age women undergoing IVF/ICSI cycles. Hum Reprod 37:1795–1805. https://doi.org/10.1093/humrep/deac114
Lin M-H, Wu FS-Y, Lee RK-K, Li S-H, Lin S-Y, Hwu Y-M (2013) Dual trigger with combination of gonadotropin-releasing hormone agonist and human chorionic gonadotropin significantly improves the live-birth rate for normal responders in GnRH-antagonist cycles. Fertil Steril 100:1296–1302. https://doi.org/10.1016/j.fertnstert.2013.07.1976
Yan M-H, Sun Z-G, Song J-Y (2023) Dual trigger for final oocyte maturation in expected normal responders with a high immature oocyte rate: a randomized controlled trial. Front Med. https://doi.org/10.3389/fmed.2023.1254982
Goodyear MDE, Krleza-Jeric K, Lemmens T (2007) The declaration of Helsinki. BMJ 335:624–625. https://doi.org/10.1136/bmj.39339.610000.BE
Alpha Scientists in Reproductive M, Embryology ESIGo (2011) The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 26:1270–1283. https://doi.org/10.1093/humrep/der037
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB (2000) Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril 73:1155–1158. https://doi.org/10.1016/S0015-0282(00)00518-5
Ben-Shlomo I, Schiff E, Levran D, Ben-Rafael Z, Mashiach S, Dor J (1991) Failure of oocyte retrieval during in vitro fertilization: a sporadic event rather than a syndrome. Fertil Steril 55:324–327. https://doi.org/10.1016/s0015-0282(16)54124-7
Schoenfeld DA (1983) Sample-size formula for the proportional-hazards regression model. Biometrics 39:499–503. https://doi.org/10.2307/2531021
Fauser BC, de Jong D, Olivennes F, Wramsby H, Tay C, Itskovitz-Eldor J, van Hooren HG (2002) Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after cotreatment with the GnRH antagonist ganirelix during ovarian hyperstimulation for in vitro fertilization. J Clin Endocrinol Metab 87:709–715. https://doi.org/10.1210/jcem.87.2.8197
Youssef MAFM, Van der Veen F, Al-Inany HG, Mochtar MH, Griesinger G, Nagi Mohesen M, Aboulfoutouh I, van Wely M (2014) Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist-assisted reproductive technology. Cochrane Database Syst Rev 2014:CD008046. https://doi.org/10.1002/14651858.CD008046.pub4
Meyer L, Murphy LA, Gumer A, Reichman DE, Rosenwaks Z, Cholst IN (2015) Risk factors for a suboptimal response to gonadotropin-releasing hormone agonist trigger during in vitro fertilization cycles. Fertil Steril 104:637–642. https://doi.org/10.1016/j.fertnstert.2015.06.011
Lin M-H, Wu FS-Y, Hwu Y-M, Lee RK-K, Li R-S, Li S-H (2019) Dual trigger with gonadotropin releasing hormone agonist and human chorionic gonadotropin significantly improves live birth rate for women with diminished ovarian reserve. Reprod Biol Endocrinol RB&E 17:7. https://doi.org/10.1186/s12958-018-0451-x
Humaidan P, Ejdrup Bredkjaer H, Westergaard LG, Yding AC (2010) 1,500 IU human chorionic gonadotropin administered at oocyte retrieval rescues the luteal phase when gonadotropin-releasing hormone agonist is used for ovulation induction: a prospective, randomized, controlled study. Fertil Steril 93:847–854. https://doi.org/10.1016/j.fertnstert.2008.12.042
Zhang J, Wang Y, Mao X, Chen Q, Hong Q, Cai R, Zhang S, Kuang Y (2017) Dual trigger of final oocyte maturation in poor ovarian responders undergoing IVF/ICSI cycles. Reprod Biomed Online 35:701–707. https://doi.org/10.1016/j.rbmo.2017.09.002
Richards JS, Ireland JJ, Rao MC, Bernath GA, Midgley AR, Reichert LE (1976) Ovarian follicular development in the rat: hormone receptor regulation by estradiol, follicle stimulating hormone and luteinizing hormone. Endocrinology 99:1562–1570. https://doi.org/10.1210/endo-99-6-1562
Yding Andersen C, Leonardsen L, Ulloa-Aguirre A, Barrios-De-Tomasi J, Moore L, Byskov AG (1999) FSH-induced resumption of meiosis in mouse oocytes: effect of different isoforms. Mol Hum Reprod 5:726–731. https://doi.org/10.1093/molehr/5.8.726
Zelinski-Wooten MB, Hutchison JS, Hess DL, Wolf DP, Stouffer RL (1995) Follicle stimulating hormone alone supports follicle growth and oocyte development in gonadotrophin-releasing hormone antagonist-treated monkeys. Hum Reprod 10:1658–1666. https://doi.org/10.1093/oxfordjournals.humrep.a136151
Eppig JJ (1979) FSH stimulates hyaluronic acid synthesis by oocyte-cumulus cell complexes from mouse preovulatory follicles. Nature 281:483–484. https://doi.org/10.1038/281483a0
Strickland S, Beers WH (1976) Studies on the role of plasminogen activator in ovulation. In vitro response of granulosa cells to gonadotropins, cyclic nucleotides, and prostaglandins. J Biol Chem 251:5694–5702
Haas J, Bassil R, Samara N, Zilberberg E, Mehta C, Orvieto R, Casper RF (2020) GnRH agonist and hCG (dual trigger) versus hCG trigger for final follicular maturation: a double-blinded, randomized controlled study. Hum Reprod 35:1648–1654. https://doi.org/10.1093/humrep/deaa107
Maged AM, Ragab MA, Shohayeb A, Saber W, Ekladious S, Hussein EA, El-Mazny A, Hany A (2021) Comparative study between single versus dual trigger for poor responders in GnRH-antagonist ICSI cycles: a randomized controlled study. Int J Gynaecol Obstet 152:395–400. https://doi.org/10.1002/ijgo.13405
Devesa M, Tur R, Rodríguez I, Coroleu B, Martínez F, Polyzos NP (2018) Cumulative live birth rates and number of oocytes retrieved in women of advanced age. a single centre analysis including 4500 women ≥38 years old. Hum Reprod 33:2010–2017. https://doi.org/10.1093/humrep/dey295
Haas J, Ophir L, Barzilay E, Machtinger R, Yung Y, Orvieto R, Hourvitz A (2016) Standard human chorionic gonadotropin versus double trigger for final oocyte maturation results in different granulosa cells gene expressions: a pilot study. Fertil Steril. https://doi.org/10.1016/j.fertnstert.2016.06.002
Park J-Y, Su Y-Q, Ariga M, Law E, Jin SLC, Conti M (2004) EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303:682–684. https://doi.org/10.1126/science.1092463
Caixeta ES, Machado MF, Ripamonte P, Price C, Buratini J (2013) Effects of FSH on the expression of receptors for oocyte-secreted factors and members of the EGF-like family during in vitro maturation in cattle. Reprod Fertil Dev 25:890–899. https://doi.org/10.1071/RD12125
Chern C-U, Li J-Y, Tsui K-H, Wang P-H, Wen Z-H, Lin L-T (2020) Dual-trigger improves the outcomes of in vitro fertilization cycles in older patients with diminished ovarian reserve: a retrospective cohort study. PLoS ONE 15:e0235707. https://doi.org/10.1371/journal.pone.0235707
Idelevich A, Vilella F (2020) Mother and embryo cross-communication. Genes. https://doi.org/10.3390/genes11040376
Law YJ, Zhang N, Venetis CA, Chambers GM, Harris K (2019) The number of oocytes associated with maximum cumulative live birth rates per aspiration depends on female age: a population study of 221 221 treatment cycles. Hum Reprod 34:1778–1787. https://doi.org/10.1093/humrep/dez100
Griesinger G, Kolibianakis EM, Papanikolaou EG, Diedrich K, Van Steirteghem A, Devroey P, Ejdrup Bredkjaer H, Humaidan P (2007) Triggering of final oocyte maturation with gonadotropin-releasing hormone agonist or human chorionic gonadotropin. Live birth after frozen-thawed embryo replacement cycles. Fertil Steril 88:616–621. https://doi.org/10.1016/j.fertnstert.2006.12.006
Peñarrubia J, Balasch J, Fábregues F, Creus M, Casamitjana R, Ballescá JL, Puerto B, Vanrell JA (1998) Human chorionic gonadotrophin luteal support overcomes luteal phase inadequacy after gonadotrophin-releasing hormone agonist-induced ovulation in gonadotrophin-stimulated cycles. Hum Reprod 13:3315–3318. https://doi.org/10.1093/humrep/13.12.3315
Nevo O, Eldar-Geva T, Kol S, Itskovitz-Eldor J (2003) Lower levels of inhibin A and pro-alphaC during the luteal phase after triggering oocyte maturation with a gonadotropin-releasing hormone agonist versus human chorionic gonadotropin. Fertil Steril 79:1123–1128. https://doi.org/10.1016/s0015-0282(03)00177-8
Ferraretti AP, La Marca A, Fauser BCJM, Tarlatzis B, Nargund G, Gianaroli L (2011) ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod 26:1616–1624. https://doi.org/10.1093/humrep/der092
Guo D, Pang C, Wang K (2024) Comparison of pregnancy outcomes in women with normal ovarian response to the gonadotropin-releasing hormone agonist protocol using different trigger methods: a single-center retrospective cohort study based on propensity score matching. Arch Gynecol Obstet 309:2153–2165. https://doi.org/10.1007/s00404-024-07404-6
Zhu S, Jiang W, Sun Y, Chen L, Li R, Chen X, Zheng B (2024) Nomogram to predict the probability of clinical pregnancy in women with poor ovarian response undergoing in vitro fertilization/ intracytoplasmic sperm injection cycles. Arch Gynecol Obstet 310:1697–1707. https://doi.org/10.1007/s00404-024-07598-9
Funding
This study was funded by the Shandong Provincial Department of Science and Technology Foundation (M-2023009).
Author information
Authors and Affiliations
Contributions
Zhijuan Wu designed the study; Minghui Yuan wrote the manuscript; Xuemei Lv developed the project; Yuying Yuan and Wen Chen conducted the data analysis; Wenhan Ju managed the data; Jingyan Song, Conghui Pang, Shuai Zhao, and Fang Lian edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
The reproductive medicine ethics committee of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine approved this study (approval no.: SZ20240813). All procedures were carried out per the relevant guidelines and regulations.
Consent to participate
Due to the retrospective design of this study and the use of anonymized data, the ethics committee approved a waiver of written informed consent from participants.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yuan, M., Lv, X., Yuan, Y. et al. Comparative analysis of hCG and dual-trigger protocols in IVF for advanced maternal age women: a single‑center retrospective cohort study based on propensity score matching. Arch Gynecol Obstet 312, 525–536 (2025). https://doi.org/10.1007/s00404-025-08037-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-025-08037-z