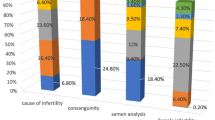
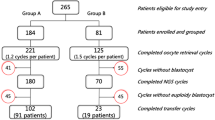
Abstract
Purpose
To study the influence of parental age on aneuploidy rates (AR) in PGT-A cycles and on the recurrence rate.
Methods
A total of 16,029 PGT-A cycles were studied over a 9-year period. The median age was 40.0 [37.0; 41.0] in women and 40.0 [37.0; 43.0] in men. In 48.3%, the biopsy was performed on day 3 embryos (D3E) and in 51.7% on blastocysts (79.5% using NGS).
Results
In women, the AR was almost constant at < 50% until the age of 35 but increased steadily to reach > 90% at 44. The AR pattern varied according to embryo stage and was considerably higher in D3E, with a steeper curve. A U-pattern was observed in D3E, whereas this was not seen in blastocysts.
In the blastocysts analyzed using NGS, trisomy 21 increased sixfold (from < 1% at < 30 to nearly 5% in women aged 40), whereas trisomies 13 and 18 increased their frequency twofold. After 3 biopsied blastocysts studied using NGS, 100% of women aged ≤ 30 had at least 1 euploid embryo, vs 96% aged 31–35, almost 80% aged 36–40, 50% aged 41–45, and 33% aged 46–50.
In terms of the man’s age, the non-adjusted analysis revealed a correlation with AR. However, after correcting for the woman’s age, no correlation was observed. The man’s age was not associated with any of the aneuploidies potentially resulting in a newborn.
Conclusions
Carrying out PGT-A systematically in IVF cycles from the age of 38–39 is highly recommended. Advanced paternal age does not carry an increased risk of aneuploidy for the embryo and does not in itself constitute an indication for PGT-A.






Similar content being viewed by others
Data availability
Data will be made available to the editors of the journal for review or query upon request.
References
Verlinsky Y, Cieslak J, Freidine M, Ivakhnenko V, Wolf G, Kovalinskaya L, White M, Lifchez A, Kaplan B, Moise J. Pregnancies following pre-conception diagnosis of common aneuploidies by fluorescent in-situ hybridization. Hum Reprod. 1995;10:1923–7.
Morales C. Current applications and controversies in preimplantation genetic testing for aneuploidies (PGT-A) in in vitro fertilization. Reprod Sci. 2024;31:66–80.
Simopoulou M, Sfakianoudis K, Maziotis E, Tsioulou P, Grigoriadis S, Rapani A, Giannelou P, Asimakopoulou M, Kokkali G, Pantou A, Nikolettos K, Vlahos N, Pantos K. PGT-A: who and when? Α systematic review and network meta-analysis of RCTs. J Assist Reprod Genet. 2021;38:1939–57.
Matorras R, Pérez-Fernández S, Mercader A, Sierra S, Larreategui Z, Ferrando M, Malaina I, Rubio C, Gantxegi M. Lessons learned from over 64,000 embryos(day 3 embryos or blastocysts) subjected to PGT-A in the same laboratory: general results, recurrence pattern and analysis of the indications. Reproductive Biomedicine Online. Published online March 27, 2024.
Gianaroli L, Magli MC, Ferraretti AP, Munne S. Preimplantation diagnosis for aneuploidies in patients undergoing in vitro fertilization with a poor prognosis: identification of the categories for which it should be proposed. Fertil Steril. 1999;72:837–8.
Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, Scott RT Jr. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101:656–63.
Platteau P, Staessen C, Michiels A, Van Steirteghem A, Liebaers I, Devroey P. Preimplantation genetic diagnosis for aneuploidy screening in women older than 37 years. Fertil Steril. 2005;84:319–24.
Kahraman S, Bahçe M, Samli H, Imirzalioğlu N, Yakisn K, Cengiz G, Dönmez E. Healthy births and ongoing pregnancies obtained by preimplantation genetic diagnosis in patients with advanced maternal age and recurrent implantation failure. Hum Reprod. 2000;15:2003–7.
Demko ZP, Simon AL, McCoy RC, Petrov DA, Rabinowitz M. Effects of maternal age on euploidy rates in a large cohort of embryos analyzed with 24-chromosome single-nucleotide polymorphism-based preimplantation genetic screening. Fertil Steril. 2016;105:1307–13.
Irani M, Cannon C, Robles A, Maddy B, Gunnala V, Qin X, Zhang C, Xu K, Rosenwaks Z. No effect of ovarian stimulation and oocyte yield on euploidy and live birth rates: an analysis of 12298 trophectoderm biopsies. Hum Reprod. 2019;35:1082–9.
Rubio C, Rodrigo L, Garcia-Pascual C, Peinado V, Campos-Galindo I, Garcia-Herrero S, Simon C. Clinical application of embryo aneuploidy testing by next-generation sequencing. Biol Reprod. 2019;101:1083–90.
Van der Scheer MW. Beiträge zur Kenntnis der Mongoloiden Missbildung (Mongolismus) auf Grund klinischer, statistischer und anatomischer Untersuchungen. (Die Bedeutung der Gebärmutterschleimhaut und des Amnions für die Aetiologie und Pathogenese dieser Missbildung). Arch NeurPsych. 1928; 19:967.
Elmerdahl Frederiksen L, Ølgaard SM, Roos L, Petersen OB, Rode L, Hartwig T, Ekelund CK; Danish Central Cytogenetics Registry Study Group; Vogel I. Maternal age and the risk of fetal aneuploidy: a nationwide cohort study of more than 500 000 singleton pregnancies in Denmark from 2008 to 2017. Acta Obstet Gynecol Scand. 2024 Feb; 103:351–359.
Mikwar M, MacFarlane AJ, Marchetti F. Mechanisms of oocyte aneuploidy associated with advanced maternal age. Mutat Res Rev Mutat Res. 2020;785: 108320.
Matorras R, Matorras F, Expósito A, Martinez L, Crisol L. Decline in human fertility rates with male age: a consequence of a decrease in male fecundity with aging? Gynecol Obstet Invest. 2011;71:229–35.
Matorras R, Malaina I, Nieto A, Limia I, Rodríguez-Gómez L. Factors influencing natural fecundity in fertile couples: a survey of puerperae and their partners. Reprod Biomed Online. Published online November 27, 2023.
Sartorius GA, Nieschlag E. Paternal age and reproduction. Hum Reprod Update. 2010;16:65–79.
Joffe M, Li Z. Male and female factors in fertility. Am J Epidemiol. 1994;140:921–9.
McPherson NO, Zander-Fox D, Vincent AD, Lane M. Combined advanced parental age has an additive negative effect on live birth rates-data from 4057 first IVF/ICSI cycles. J Assist Reprod Genet. 2018;35:279–87.
Kühnert B, Nieschlag E. Reproductive functions of the ageing male. Hum Reprod Update. 2004;10:327–39.
Hellstrom WJ, Overstreet JW, Sikka SC, Denne J, Ahuja S, Hoover AM, Sides GD, Cordell WH, Harrison LM, Whitaker JS. Semen and sperm reference ranges for men 45 years of age and older. J Androl. 2006;27:421–8.
Orioli IM, Castilla EE, Scarano G, Mastroiacovo P. Effect of paternal age in achondroplasia, thanatophoric dysplasia, and osteogenesis imperfecta. Am J Med Genet. 1995;59:209–17.
Toriello HV, Meck JM; Professional Practice and Guidelines Committee. Statement on guidance for genetic counseling in advanced paternal age. Genet Med. 2008; 10:457–60.
Carrasquillo RJ, Kohn TP, Cinnioglu C, Rubio C, Simon C, Ramasamy R, Al-Asmar N. Advanced paternal age does not affect embryo aneuploidy following blastocyst biopsy in egg donor cycles. J Assist Reprod Genet. 2019;36:2039–45.
Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet. 2013;13:493–504.
Kubicek D, Hornak M, Horak J, Navratil R, Tauwinklova G, Rubes J, Vesela K. Incidence and origin of meiotic whole and segmental chromosomal aneuploidies detected by karyomapping. Reprod Biomed Online. 2019;38:330–9.
Martin RH, Rademaker A. The frequency of aneuploidy among individual chromosomes in 6,821 human sperm chromosome complements. Cytogenet Cell Genet. 1990;53:103–7.
Templado C, Vidal F, Estop A. Aneuploidy in human spermatozoa. Cytogenet Genome Res. 2011;133:91–9.
Bonus ML, McQueen DB, Ruderman R, Hughes L, Merrion K, Maisenbacher MK, Feinberg E, Boots C. Relationship between paternal factors and embryonic aneuploidy of paternal origin. Fertil Steril. 2022;118:281–8.
Zaragoza MV, Jacobs PA, James RS, Rogan P, Sherman S, Hassold T. Nondisjunction of human acrocentric chromosomes: studies of 432 trisomic fetuses and liveborns. Hum Genet. 1994;94:411–7.
Fonseka KG, Griffin DK. Is there a paternal age effect for aneuploidy? Cyto-genet Genome Res. 2011;133:280–91.
Rodrigo L, Clemente-Císcar M, Campos-Galindo I, Peinado V, Simón C, Rubio C. Characteristics of the IVF cycle that contribute to the incidence of mosaicism. Genes(Basel). 2020; 11:1151.
Eskenazi B, Wyrobek AJ, Sloter E, Kidd SA, Moore L, Young S, Moore D. The association of age and semen quality in healthy men. Hum Reprod. 2003;18:447–54.
Asada H, Sueoka K, Hashiba T, Kuroshima M, Kobayashi N, Yoshimura Y.The effects of age and abnormal sperm count on the nondisjunction of spermatozoa .J Assist Reprod Genet.2000; 17:51–9.
Griffin DK, Abruzzo MA, Millie EA, Sheean LA, Feingold E, Sherman SL, Hassold TJ. Non-disjunction in human sperm: evidence for an effect of increasing paternal age. Hum Mol Genet. 1995;4:2227–32.
Garcia-Velasco JA, Bermejo A, Ruiz F, Martinez-Salazar J, Requena A, Pellicer A. Cycle scheduling with oral contraceptive pills in the GnRH antagonist protocol vs the long protocol: a randomized, controlled trial. Fertil Steril. 2011;96:590–3.
Labarta E, Bosch E, Mercader A, Alamá P, Mateu E, Pellicer A. A higher ovarian response after stimulation for IVF is related to a higher number of euploid embryos. Biomed Res Int. 2017;2017:5637923.
ASEBIR Special Interest Group of Embryology. Cuadernos de Embriología Clínica. Criterios ASEBIR de valoración morfológica de oocitos, embriones tempranos y blastocistos humanos. 2nd ed, 2008. ASEBIR, Madrid.
Likas A, Vlassis N, Verbeek JJ. The global k-means clustering algorithm. Pattern Recogn. 2003;36:451–546.
Rijnders PM, Jansen CA. The predictive value of day 3 embryo morphology regarding blastocyst formation, pregnancy and implantation rate after day 5 transfer following in-vitro fertilization or intracytoplasmic sperm injection. Hum Reprod. 1998;13:2869–73.
Yin H, Jiang H, He R, Wang C, Zhu J, Luan K. The effects of fertilization mode, embryo morphology at day 3, and female age on blastocyst formation and the clinical outcomes. Syst Biol Reprod Med. 2015;61:50–6.
Langley MT, Marek DM, Gardner DK, Doody KM, Doody KJ. Extended embryo culture in human assisted reproduction treatments. Hum Reprod. 2001;16:902–8.
Barbash-Hazan S, Frumkin T, Malcov M, Yaron Y, Cohen T, Azem F, Amit A, Ben-Yosef D. Preimplantation aneuploid embryos undergo self-correction in correlation with their developmental potential. Fertil Steril. 2009;92:890–6.
Gruhn JR, Zielinska AP, Shukla V, Blanshard R, Capalbo A, Cimadomo D, Nikiforov D, Chan AC, Newnham LJ, Vogel I, Scarica C, Krapchev M, Taylor D, Kristensen SG, Cheng J, Ernst E, Bjørn AB, Colmorn LB, Blayney M, Elder K, Liss J, Hartshorne G, Grøndahl ML, Rienzi L, Ubaldi F, McCoy R, Lukaszuk K, Andersen CY, Schuh M, Hoffmann ER. Chromosome errors in human eggs shape natural fertility over reproductive life span. Science. 2019;365:1466–9.
Wyrobek AJ, Aardema M, Eichenlaub-Ritter U, Ferguson L, Marchetti F. Mechanisms and targets involved in maternal and paternal age effects on numerical aneuploidy. Environ Mol Mutagen. 1996;28:254–64.
Heiser HC, Cagnin NF, de Souza MU, Ali TM, Estrada PRQ, de Souza CCWD, Coprerski B, Rubio C, Riboldi M. The embryo mosaicism profile of next-generation sequencing PGT-A in different clinical conditions and their associations. Front Reprod Health. 2023;5:1132662.
Nakhuda G, Jing C, Butler R, Guimond C, Hitkari J, Taylor E, Tallon N, Yuzpe A. Frequencies of chromosome-specific mosaicisms in trophoectoderm biopsies detected by next-generation sequencing. Fertil Steril. 2018;109:857–65.
Currie CE, Ford E, Benham Whyte L, Taylor DM, Mihalas BP, Erent M, Marston AL, Hartshorne GM, McAinsh AD. The first mitotic division of human embryos is highly error prone. Nat Commun. 2022;13:6755.
Newberger DS. Down syndrome: prenatal risk assessment and diagnosis. Am Fam Physician. 2000;62:825–38.
Zhang XH, Qiu LQ, Ye YH, Xu J. Chromosomal abnormalities: subgroup analysis by maternal age and perinatal features in zhejiang province of China, 2011–2015. Ital J Pediatr. 2017;43:47.
Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev. 2015;19:22–33.
Jimbo M, Kunisaki J, Ghaed M, Yu V, Flores HA, Hotaling JM. Fertility in the aging male: a systematic review. Fertil Steril. 2022;118:1022–34.
Lowe X, Eskenazi B, Nelson DO, Kidd S, Alme A, Wyrobek AJ. Frequency of XY sperm increases with age in fathers of boys with Klinefelter syndrome. Am J Hum Genet. 2001;69:1046–54.
Sharma R, Agarwal A, Rohra VK, Assidi M, Abu-Elmagd M, Turki RF. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod Biol Endocrinol. 2015;13:35.
Ramasamy R, Chiba K, Butler P, Lamb DJ. Male biological clock: a critical analysis of advanced paternal age. Fertil Steril. 2015;103:1402–6.
Auger J, Jouannet P. Age and male fertility: biological factors. Rev Epidemiol Sante Publique. 2005;53:25–35.
García-Ferreyra J, Luna D, Villegas L, Romero R, Zavala P, Hilario R, Dueñas-Chacón J. High aneuploidy rates observed in embryos derived from donated oocytes are related to male aging and high percentages of sperm DNA fragmentation. Clin Med Insights Reprod Health. 2015;9:21–7.
García-Ferreyra J, Hilario R, Dueñas J. High percentages of embryos with 21, 18 or 13 trisomy are related to advanced paternal age in donor egg cycles. JBRA Assist Reprod. 2018;22:26–34.
Dviri M, Madjunkova S, Koziarz A, Antes R, Abramov R, Mashiach J, Moskovtsev S, Kuznyetsova I, Librach C. Is there a correlation between paternal age and aneuploidy rate? An analysis of 3,118 embryos derived from young egg donors. Fertil Steril. 2020;114:293–300.
Samarasekera T, Willats E, Green MP, Hardy T, Rombauts L, Zander-Fox D. Impact of male age on paternal aneuploidy: single-nucleotide polymorphism microarray outcomes following blastocyst biopsy. Reprod Biomed Online. 2023;47: 103245.
Aknowledgements
The author I.M. was supported by Basque Government funding, grant IT456-22.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 931 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matorras, R., Sierra, S., Pérez-Fernández, S. et al. Influence of parental age on chromosomal abnormalities in PGT-A embryos: exponentially increasing in the mother and completely null in the father. J Assist Reprod Genet 42, 1833–1844 (2025). https://doi.org/10.1007/s10815-025-03462-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-025-03462-0