Abstract
Purpose
Elevated progesterone (P4) has emerged as a cofounder for embryo quality and recently been investigated in blastocyst aneuploidy rates. In this context, we explored the prevalence of aneuploidy in blastocysts generated from donated eggs according to P4 levels on trigger’s day.
Methods
This retrospective cohort study analyzed data from intracytoplasmic sperm injection (ICSI) cycles using frozen donated oocytes that underwent embryo biopsy (PGT-A) at blastocyst stage. Patients were divided into two groups according to serum P4 on trigger day: < 1.5 ng/mL (group A) and ≥ 1.5 ng/mL (group B). Only euploid embryos were transferred to recipients. Primary outcome was embryo euploidy and aneuploidy rate. Secondary outcomes were number of blastocysts, number of top-quality embryos, number of euploid/aneuploid embryos and clinical pregnancy rate.
Results
259 ICSI PGT-A cycles with frozen donated oocytes were analyzed. Group A included 75 cycles (57 donors; 69 recipients) and group B 184 cycles (115 donors; 163 recipients). The number of blastocysts (3.60 ± 1.52 vs 3.68 ± 1.52, P = 0.667), top-quality embryos (2.27 ± 1.59 vs 2.28 ± 1.43, P = 0.802), euploid embryos (1.92 ± 1.25 vs 1.92 ± 1.13, P = 0.954) and aneuploid embryos (1.23 ± 1.01 vs 1.14 ± 0.94, P = 0.593) were not significantly different between groups A and B, respectively. Euploid embryo rate (A: 0.31 ± 0.20 vs B: 0.30 ± 0.18, P = 0.626), aneuploidy embryo rate (A: 0.21 ± 0.19 vs B: 0.18 ± 0.15, P = 0.436) and clinical pregnancy rate (A: 73% vs B: 82%, P = 0.476) were comparable between the two groups.
Conclusions
Elevated P4 values on trigger day did not affect embryo ploidy or embryo quality parameters in donated eggs cycles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
In this study we found that serum progesterone on trigger day did not affect embryo ploidy or embryo quality parameters in donated egg cycles. In an era marked by cycles with freeze all, PGT-A and FET, in which the percentage of IVF treatments using donated eggs only grows, our data should be of important interest to the Human Reproduction community worldwide and in relevance for planning and improving egg donation cycles, with benefits for the infertile couple. |
Introduction
Chromosome aneuploidy in human embryos, whether they are generated by natural conception or by assisted reproductive technologies (ART), is the main cause for failed implantation, pregnancy loss and congenital birth defects [1]. The vast majority of aneuploidies are caused by errors in chromosomal/chromatid segregation during maternal meiosis and are known to be maternal age-related [2]. The incidence of aneuploidies increases from approximately 30% in patients younger than 35 years to 80% in women 42 years and older [3]. These chromosomal abnormalities can also occur after fertilization, during meiosis resumption and subsequent mitoses [4], leading to mosaicism in embryos. These errors can result in aneuploid embryos or embryos with chromosomal mosaicism.
Despite the high frequency of human aneuploidy in older women and its clinical importance [5], the mechanisms underlying aneuploidy are still poorly understood. Apart from maternal age, the exact cause of these numeric imbalances it is not known.
Over the years, many studies have tried to answer the question whether other factors involved in in vitro fertilization (IVF) treatments could interfere with the normal physiology of the oocyte maturation, fertilization process, subsequent embryonic development and, therefore, increase aneuploidy rates in embryos. Ovarian stimulation protocols [6, 7], fertilization method [8] and laboratory conditions [9], such as embryo culture media, pH, oxygen, osmolarity and temperature, have been proposed as possible candidates to increase meiotic errors. The documented variability in the frequency of aneuploid embryos between fertility centers [10] suggests that these IVF variables might contribute to the incidence of aneuploidy.
Since P4 plays a key role in the reproductive process and elevated P4 occurs in up to 46% of stimulated cycles [11], recently, the influence of high P4 values in IVF cycles and its possible detrimental effect on embryo ploidy have been investigated [12,13,14].
Studies have already demonstrated the presence of membrane receptors with high affinity for P4 in mammalian oocytes, including humans, and their ability to bind to P4 [15]. In recent years, compelling evidence has shown that the P4 receptor membrane component 1 (PGRMC1) is expressed in many cells of the mammalian reproductive system, where it exerts diverse functions [16]. In the ovary, PGRMC1 affects follicular growth by controlling cell viability and granulosa’s proliferation. PGRMC1 also appears to have a direct role in promoting the proper completion of oocyte maturation and in early embryonic development [16].
In animal models, it has been demonstrated that PGRMC1 may have an important role in oocyte maturation, more specifically related to the mechanism of chromosome segregation and polar body extrusion [16]. The mechanism by which PGRMC1 controls mitotic and meiotic cell division appears to involve an association with the cell spindle apparatus. According to recent analyzes and data, PGRMC1 participates in key mechanisms that control the progression of meiosis, such as spindle formation, stabilization of cytoskeletal elements, faithful separation of bivalent chromosomes, and cytokinesis, processes that are essential for proper maturation of the oocyte [16]. These findings suggest that alterations in the activity of the PGRMC1 could account in part for low oocyte development competence, reflecting at increased errors in chromosome segregation and, consequently, in aneuploid embryos.
Based on experimental evidence and clinical data, our hypothesis is that extra-physiological P4 levels in IVF ovarian stimulation could interfere with oocyte maturation via specific oocyte membrane receptors, leading to inadequate segregation of chromosomes during meiosis and, consequently, increasing embryo aneuploidy. This study aimed to explore if P4 levels on the day of the trigger affect the prevalence of aneuploidy in embryos generated from donated eggs in IVF cycles.
Material and methods
Study design
This is a retrospective single-center cohort study that analyzed 259 intracytoplasmic sperm injection (ICSI) cycles using frozen donated oocytes that underwent PGT-A at blastocyst stage performed between April/2013 and February/2019. Patients were divided in two groups according to P4 value on day of trigger: group A if P4 < 1.5 ng/mL and group B if P4 ≥ 1.5 ng/mL. A threshold P4 value of 1.5 ng/mL was chosen based on published literature [17]. Only euploid embryos were transferred to recipients. Primary outcome was embryo euploidy and aneuploidy rate. Secondary outcomes were number of blastocysts, number of top-quality embryos, number of euploid/aneuploid embryos and clinical pregnancy rate. Since it is already well documented that serum P4 on trigger is significantly correlated with the number of follicles and number of oocytes [13], in order to adjust the results of the studied outcomes, we calculated the P4/AFC index of the donors. Two new groups were created using the median of the P4/AFC index as a cut-off point: group 1 presented a P4/AFC index < 0.1 (n = 126 cycles) and group 2 presented a P4/AFC index ≥ 0.1 (n = 133 cycles).
Patient selection
Oocyte donors
Donors were women between 18 and 35 years of age, with regular menstrual cycles, body mass index (BMI) under 30 kg/m2, normal karyotype, with no gynecological or other health diseases and antral follicular count (AFC) ≥ 12. They also had no family history of hereditary or chromosomal diseases and were not exposed to radiation, hazardous chemical substances or intravenous drugs. All donors included in this study joined our oocyte program between April/2013 and February/2019. All of them underwent ovarian stimulation during this time interval.
Due to high ovarian reserve, a single donor, after ovarian stimulation, was able to donate their eggs to more than one recipient. Since in Brazil egg donation is anonymous and there is no financial compensation for donors, it is common for a donor to donate her eggs to more than one couple, especially considering the increasing number of cycles using donated eggs all around the world over the years.
Oocyte recipients
Oocyte recipients were admitted in our oocyte donation program due to advanced maternal age, premature ovarian failure or low response to previous stimulation. Recipients chose to perform preimplantation genetic testing (PGT-A) in oocyte donors’ cycles because of their own personal reasons; it was not due to medical recommendation. It is worth to mention that according to Brazilian law, with exception to sexual violence and/or maternal health risk, pregnancy interruption is forbidden by social/psychological reasons. Each recipient received a quantity of 8–12 eggs in order to optimize the chance of pregnancy and live birth rates [18].
Exclusion criteria
Cases with severe male infertility (< 5 million of fresh spermatozoa) were not included in the present study.
Laboratory procedures
Stimulation protocol
All oocyte donors received standard ovarian stimulation protocol with GnRH antagonist. Recombinant follicle-stimulating hormone (rFSH) or human menopausal gonadotropin (hMG) was administered starting on day 2–3 of the menstrual cycle. The dose of gonadotropin was individualized for each patient according to AFC and follicular development during stimulation. When a lead follicle reached 13 mm in diameter, the GnRH antagonist was initiated. The GnRH antagonist was administered daily until the day of trigger. Final oocyte maturation was induced when at least two lead follicles reached 20 mm in diameter with GnRH agonist. Thirty-five to thirty-six hours after trigger, the oocytes were collected via transvaginal ultrasound guidance. Mature oocytes (MII) were then cryopreserved.
Embryo biopsy
Post-thaw mature oocytes were fertilized using ICSI. At our center, incubation conditions are set at 37 °C; 6% CO2; 5% O2. Embryo development was evaluated until the day of biopsy. We used the morphologic criteria established by the Istanbul Consensus to classify the embryos. The scoring system for blastocysts was a combination of the stage of development and the grade of the inner cell mass and the trophectoderm [19].
Embryo biopsy was performed at blastocyst stage, on days 5 or 6 of development, using laser technology. Three to eight cells from the trophectoderm were collected to perform PGT-A. The samples were analyzed by array comparative hybridization technology (CGH) or next-generation sequencing (NGS). ACGH was the method used in our center in 2013, 2014 and 2015 and NGS was introduced in 2016. Embryos were classified as euploid (46, XX or 46, XY) or aneuploid (if they have an extra or missing chromosome). Mosaicism and other chromosome abnormalities were considered as aneuploid.
Embryo transfer
Frozen embryo transfer (FET) cycle preparation was performed with hormone replacement treatment. Only euploid embryos were transferred to recipients.
Progesterone measurement
Donor serum P4 was measured on trigger day using chemiluminescence immunoassay (Elecsys Progesterone III, Roche Diagnostics). The sensitivity of the assay was 0.03 μg/L. All donors underwent blood tests at Cerba laboratory to avoid bias.
Outcomes
The primary outcome was embryo euploidy and aneuploidy rate. Secondary outcomes were the number of blastocysts available for biopsy, number of top-quality embryos, number of euploid/aneuploid embryos and clinical pregnancy rate.
Statistical analysis
Statistical analysis was performed with SPSS version 17. Quantitative results are presented as mean and standard deviation and frequencies, percentages for qualitative data. After the evaluation of the normal distribution or not of quantitative data, comparisons were performed with Student’s T test (parametric test for normal data) or the Mann–Whitney U test (non-parametric test for non-normal data). All rates are presented as mean values except for clinical pregnancy rate which is presented as n (%). For all calculations, a P value of < 0.05 was considered as statistically significant. According to statistical analysis, the minimum number of participants per group is 24. The sampling power of our study is 99.8%.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki and approved by the Ethical Committee of Clinical Research at Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (CAAE number 40900820.5.0000.0068).
Results
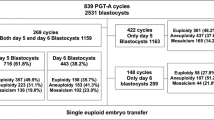
A total of 259 ICSI PGT-A cycles with frozen donated oocytes were included in this study. Based on P4 value on the day of trigger, 75 cycles (29.0%) presented serum P4 < 1.5 ng/m (group A) and 184 cycles (71.0%) presented serum P4 ≥ 1.5 ng/mL (group B). Group A included 57 donors and 69 recipients and Group B included 115 donors and 163 recipients.
Donors’ cycles
Donors’ baseline characteristics and ovarian stimulation outcomes are summarized in Table 1. Donors’ age ranged from 18 to 34 years and was statistically similar between the two groups. Average donor age was 25.15 ± 3.59 years for group A and 24.46 ± 3.73 years for group B (P = 0.190). Group B had a higher AFC than Group A (22.95 ± 10.65 vs 19.60 ± 7.08, P = 0.030).
It is already well documented that serum P4 levels on trigger during IVF cycles are attributed to an excessive number of follicles, each one producing a normal amount of P4 during follicular development [11]. In other words, the greater the number of follicles and oocytes, the higher are P4 levels. As a result, premature P4 elevation is a commonly observed phenomenon in donor’s cycles during controlled ovarian hyperstimulation due to the high ovarian reserve of donor patients [20]. In our study, since Group B presented a higher AFC, it included a greater number of cycles in its sample.
Regarding the ovarian stimulation cycle, total gonadotropin dosage (2777.83 ± 526.02 IU vs 2814.54 ± 538.78 IU, P = 0.736) and basal FSH (5.19 ± 1.51 IU/L vs 5.22 ± 1.47 IU/L, P = 0.303) were not different between groups A and B, respectively. Estradiol levels on the day of trigger (5255.00 ± 6405.77 pg/mL vs 5252.83 ± 4346.33 pg/mL, P = 0.034), number of retrieved eggs (33.96 ± 14.39 vs 28.01 ± 11.78, P = 0.001) and number of mature oocytes (24.84 ± 11.74 vs 21.12 ± 10.56, P = 0.008) were significantly higher in Group B (probably due to a higher AFC). There was no difference in the MII rate between the two groups (A: 0.75 ± 0.16 vs B: 0.73 ± 0.16, P = 0.071).
Recipients’ cycles
Average recipient age was 43.04 ± 4.47 years for group A and 42.27 ± 3.56 years for group B (P = 0.189). Both groups had similar BMI (23.94 ± 3.57 vs 23.52 ± 3.68 k/m2, P = 0.575). Outcomes of recipients’ cycles are presented in Table 2. There were no differences in the number of fertilized oocytes (A: 6.28 ± 1.45 vs B: 6.61 ± 1.59, P = 0.168) or fertilization rate (A: 0.83 ± 0.14 vs B: 0.82 ± 0.13, P = 0.854) between the two groups.
Regarding embryo development, number of blastocysts (3.60 ± 1.52 vs 3.68 ± 1.52, P = 0.667), number of biopsied blastocyst (3.15 ± 1.33 vs 3.06 ± 1.29, P = 0.699) and number of top-quality embryos (2.27 ± 1.59 vs 2.28 ± 1.43, P = 0.802) were found not to be significantly different between groups A and B, respectively. Seminal parameters were similar between the two groups.
A total of 155 blastocysts (16.35%) were analyzed by CGH and 793 blastocysts (77.95%) were analyzed by NGS. Numbers of euploid embryos (A: 1.92 ± 1.25 vs B: 1.92 ± 1.13, P = 0.954), euploid embryo rate (A: 0.31 ± 0.20 vs B: 0.30 ± 0.18, P = 0.626), and aneuploid embryos (A: 1.23 ± 1.01 vs B: 1.14 ± 0.94, P = 0.592) and aneuploid embryo rate (A: 0.21 ± 0.19 vs B: 0.18 ± 0.15, P = 0.436) were not significantly different in the two groups.
FET outcomes and pregnancy results
The mean of endometrial thickness during FET was 8.71 ± 1.68 mm, which was not different between the two groups (A: 8.98 ± 1.56 mm vs B: 8.64 ± 1.71 mm, P = 0.365). In group A, we had 15 cycles in which the biopsied embryos were transferred to recipients; and in group B, we had 50 cycles. There was no difference in clinical pregnancy rate (A: 73% vs B: 82%, P = 0.476).
Donors and recipients cycles’ outcomes adjusted by the P4/AFC index
To adjust the results of the studied outcomes, we calculated the P4/AFC index of the donors. Two new groups were created using the median of the P4/AFC index as a cut-off point. As exposed in Table 3, Group 1 presented a P4/AFC index < 0.1 (n = 126 cycles) and Group 2 presented a P4/AFC index ≥ 0.1 (n = 133 cycles).
In donors’ ovarian stimulation cycles, after calculating this index (Table 4), no significant difference was found between groups 1 and 2 regarding total of used gonadotropins (2769.74 ± 528.96 vs 2836.74 ± 541,45 IU, P = 0.448), basal FSH (5.20 ± 1.78 vs 5.29 ± 1.48 IU/L, P = 0.193), estradiol at trigger 4983.30 ± 3846.34 vs 5549.15 ± 3846.34 pg/mL, P = 0.351), number of retrieved eggs (32.75 ± 13.36 vs 31.77 ± 14.53, P = 0.421), number of MII (24.40 ± 10.86 vs 23.17 ± 12.15, P = 0.188) and MII rate (0.75 ± 0.13 vs 0.72 ± 0.15, P = 0.184).
After adjusting the statistical analysis according to donors’ AFC, we did not find significant differences between the two groups regarding estradiol at trigger, number of eggs retrieved and number of MII, as we compare Tables 1 and 4.
Regarding recipients’ cycles, after calculating the P4/AFC index (Table 5), we did not observe a significant difference between Groups 1 and 2 in relation to fertilization rate (0.84 ± 0.013 vs 0.81 ± 0.14, P = 0.135), number of blastocysts (3.70 ± 1.47 vs 3.62 ± 1.57, P = 0.623) and number of top-quality embryos (2.25 ± 1.47 vs 2.31 ± 1.47, P = 0.821). Numbers of euploid (2.01 ± 1.22 vs 1.83 ± 1.11, P = 0.288) and aneuploid (1.19 ± 0.99 vs 1.14 ± 0.93, P = 0.674) embryos were similar among groups. There was also no difference in euploidy rate (0.32 ± 0.19 vs 0.28 ± 0.17, P = 0.129) and aneuploidy rate (0.19 ± 0.99 vs 1.14 ± 0.93, P = 0.674). Clinical pregnancy rate was similar in groups 1 and 2 (73% vs 82%, P = 0.476) as shown in Table 5.
After adjusting the statistical analysis according to donors’ AFC, we continued to observe no differences between the two groups regarding the analyzed parameters related to fertilization, blastocyst formation, embryo ploidy and clinical pregnancy rate as we compare Tables 2 and 5.
Discussion
Late follicular phase P4 elevation (LFPE) occurs in up to 46% of patients who undergo IVF stimulation [11]. The effect of this premature P4 elevation on clinical and laboratory results of IVF treatments continues to be a theme of debate during the past decades. In an era of freeze-all, PGT-A and FET cycles, with an increasing number of treatments using donated eggs, understanding the effects of serum P4 on embryo quality and ploidy is extremely important, especially in egg donated cycles, in which LFPE is a commonly observed phenomenon during controlled ovarian hyperstimulation due to a high ovarian reserve of these patients.
To our knowledge, this is the first study to evaluate the influence of P4 on embryo ploidy in egg donated cycles. Our findings suggest that elevated donor P4 values on day of trigger may not affect embryo ploidy or embryo euploidy rate in ICSI PGT-A cycles using frozen donated oocytes. We also found that P4 values on day of trigger were not associated with impaired embryo development or worst blastocyst quality parameters in donated egg cycles. Further, high P4 did not show a significant influence on clinical pregnancy following the transfer of euploid embryos to recipients.
Over the years, several studies have described the association between elevated P4 and pregnancy rates. Schoolcraf et al. [21] were the first to report that clinical pregnancy was negatively influenced by serum P4 on the day of hCG administration. These findings were subsequently supported by other studies in the last two decades [11, 17, 22]. The main hypothesis to explain these results is the detrimental effect of high P4 levels on endometrial receptivity. The authors have found that patients with high P4 on trigger day demonstrate a difference in endometrial gene expression and implantation regulating proteins during the traditional window of implantation [23,24,25]. Therefore, it is likely that asynchrony between the embryo and the endometrium in patients with elevated P4 is the mechanism explaining lower pregnancy rates in women undergoing fresh autologous cycles. This assumption was further supported by Shapiro et al. [26] and Baldini et al. [27], who found that P4 elevation on the day of hCG administration did not affect the outcomes of IVF/pregnancy rates after freeze-all strategy followed by FET.
Our data show that P4 elevation did not have a negative impact on ongoing pregnancy rates in egg donated cycles. In our study, by combining PGT-A with FET in oocyte donated cycles, we were able to control the influence of P4 on the endometrium, thus suggesting a deleterious effect of P4 in the endometrium and not in oocyte/embryo quality. A number of other studies performed in oocyte donation programs support the findings we report here. These researches suggest that pregnancy rates of recipients were not negatively influenced by P4 levels of the donors on day of trigger [28,29,30].
Although the detrimental effect of high P4 levels on endometrial physiology and receptivity is well established in the published literature, controversy continues to exist regarding the effect of elevated P4 on oocyte and embryo quality. Some studies that evaluated the impact of supraphysiological P4 on embryonic quality through morphological classification suggested that embryo quality was negatively correlated with P4 levels during ovarian stimulation [31,32,33]. However, it is important to highlight that the generalization of these results is problematic since these studies analyzed embryos at cleavage stage and/or had a small sample for statistical analysis [31]. Our study, on the other hand, only included in its analysis frozen oocytes that where fertilized and that developed to blastocyst stage. Our data showed no association between high P4 and oocyte maturity rate, fertilization rate, number of blastocysts and number of top-quality embryos, suggesting that elevated P4 did not have a negative impact on embryo development nor on blastocyst quality. Our results are consistent with several other studies that also have not reported a negative association between P4 and embryo quality [20, 22, 34,35,36,37,38]. Our findings are also in agreement with other studies performed in oocyte donation programs that did not demonstrate an association between P4 and embryo quality [20, 28, 29].
This inconsistency regarding P4 and oocyte/embryo quality may be due to different P4 cut-off values and discrepancies regarding P4 measurements. A recent meta-analysis of 63 studies involving over 60.000 cycles [11] showed that the P4 threshold value on day of trigger and the methodology used for P4 mensuration varied among the papers. Although there is no consensus on the P4 cut-off, a serum P4 level of 1.5 ng/ml on the day of hCG administration seems to be the most consensual threshold to define detrimental levels of P4 for the outcome of IVF/ICSI–ET cycles [17]. Therefore, for our analysis, a cut-off value of 1.5 ng/mL was adopted. Since there is difficulty in interpreting and comparing results between different studies due to multiple factors directly associated with the method of measuring P4, for our analysis, we sought to eliminate this confounding bias by evaluating all P4 samples in the same laboratory, using a reliable assay that was consistently used under identical conditions and within the same relative time interval when monitoring donors’ ovarian stimulation.
Another point of controversy regarding the effect of P4 and oocyte/embryo quality is the discrepancy between P4 serum levels and intra-follicular values [38]. Shufaro et al. [39] discussed the origin of elevated blood P4 and proposed a new index to measure it based on average P4 secretion per follicle rather than total blood P4. These considerations raise the question that may be P4 intra-follicular measurement per follicle is a more appropriate method than blood measurement since this is a representation of the intrinsic follicular properties [39]. Since it is already well documented that serum P4 level on trigger day in IVF cycles is significantly correlated with the number of follicles and number of oocytes [13], our outcomes were analyzed after correcting the statistical results considering the AFC of the donors in order to avoid bias.
The influence of P4 on embryo ploidy is a topic that only recently has been questioned. Experimental evidence demonstrates the presence of membrane receptors with high affinity for P4 (PGRMC1) in many cells of the mammalian reproductive system [15]. In the ovary, studies on animals support the hypothesis that PGMRC1 affects oocyte biology both extrinsically through the regulation of follicular growth, and intrinsically, during oocyte maturation [16]. According to a recent review in bovine oocytes, PGRMC1 appears to participate in key mechanisms that control the progression of meiosis and that are essential at promoting the adequate completion of oocyte maturation [16]. Evidence suggests that these receptors also have a role in regulating early embryonic development [16]. Such findings suggest an important role of P4, via the PGRMC1 receptor, in oocyte maturation, more specifically related to the mechanism of chromosome segregation during meiosis [16].
Based on experimental evidence, the hypothesis of our study was that extra-physiological P4 during ovarian stimulation in IVF could interfere with oocyte maturation, via specific membrane receptors present in oocytes, leading to a higher rate of aneuploidy. Despite these findings in animal models, our study showed no deleterious effect of high P4 in human embryo ploidy. Our results suggest that possible disturbances in P4 values do not influence the final stages of oocyte maturation and do not have an impact in the meiotic process by increasing the risk of a non-disjunction error via specific oocyte membrane receptors. Our results are consistent with recent studies [12,13,14] that also describe no correlation between P4 values and human embryo ploidy. Due to these differences in results between human and animal models, future studies evaluating gene expression pathways and the molecular dynamics of human embryo division in a non-physiological hormonal environment during ovarian stimulation are most needed to evaluate the real effect of premature P4 elevation on oocyte fertilization, embryonic development and reproductive potential on humans.
A limitation of the existing studies in literature that evaluated the relationship between serum P4 and human embryo ploidy [12,13,14] is the fact that none of them included in their design the analysis of oocyte donors. Thus, they investigated a heterogeneous IVF population. Their results come from embryos generated from infertile women, whose oocyte quality could be impaired due to different types of infertility. On the other hand, our data focused on donors, presumably young fertile women, in whom we expect good-quality oocytes and low chromosome abnormalities rates [10]. Another limitation of the studies [12,13,14] that analyzed the effect of serum P4 levels on human embryo ploidy is the fact that the authors only compared the results using the adopted P4 cut-off value. Contrary to this, to avoid bias, our analysis was adjusted by comparing the groups according to the P4/AFC index.
Furthermore, studies using egg donors as a control population have demonstrated that rates of preimplantation embryo aneuploidy vary between different IVF centers by up to 40% [10]. This suggests that site-specific conditions, in the clinic, in IVF techniques, in the laboratory or in the used medications for ovarian stimulation, could influence chromosomal dynamics [9]. Variables including culture and medium, pH, temperature, osmolarity, and oxygen concentration may all be possible factors influencing chromosomal integrity [9]. Also, laboratory techniques, insemination method, use of laser or manipulation of biopsied cells could also influence genetic results [13]. Since some studies [9, 10] have suggested that factors involved in IVF treatments could interfere with oocyte maturation, embryo development and aneuploidies rates, our study was conducted in a single center, where laboratory conditions, ovarian stimulation protocol, fertilization method and embryo biopsy technique were the same in all analyzed cycles.
Our study is not without limitations. This is a retrospective study were conclusions were taken based on observational data, although we only included PGT-A followed by FET in oocyte donated cycles in order to reduce the chances of bias. Another limitation was the fact that we used two PGT-A methods on trophectoderm biopsy: CGH and NGS. Although we had a little number of biopsied embryos analyzed by CGH, literature shows that little discrepancies may exist when comparing the two technologies [40].
Conclusion
In summary, our study suggests that elevated P4 values on trigger day did not affect embryo ploidy or embryo quality parameters in egg donated cycles. Multicentric studies, with a larger sample, would be desirable to confirm our findings. Considering the increasing number of cycles using donated oocytes over the years, our data should be of interest to the ART community. Furthermore, future studies evaluating the molecular dynamics of embryo division during ovarian stimulation in IVF treatments should elucidate the real effect of premature P4 elevation on oocyte maturation and embryonic development. In this context, the proposal of a new evaluation index based on intra-follicular P4 and not just serum P4 is also an important point to be considered.
Finally, more studies correlating different factors involved in IVF treatments and embryo ploidy are needed to fully understand other causes of aneuploidy rather maternal age. Despite the high frequency of human aneuploidy in older women and its clinical and economic importance, the mechanisms involved in chromosomal abnormalities are not completely known. This elucidation should provide opportunities for improving laboratory and clinical IVF outcomes.
Data availability
All required information regarding the study protocol and collected data will be made available upon reasonable request. Proposals shoud be directed to pri.lcaldeira@hotmail.com.
References
Nagaoka SI, Hassold TJ, Hunt PA (2012) Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet 13(7):493–504
Hassold T, Hunt P (2001) To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2(4):228–291
Demko ZP, Simon AL, McCoy RC, Petrov DA, Rabinowitz M (2016) Effects of maternal age on euploidy rates in a large cohort of embryos analyzed with 24-chromosome single-nucleotide polymorphism-based preimplantation genetic screening. Fertil Steril 105(5):1307–1313
Munné S, Chen S, Colls P, Garrisi J, Zheng X, Cekleniak N, Lenzi M, Hughes P, Fischer J, Garrisi M, Tomkin G, Cohen J (2007) Maternal age, morphology, development and chromosome abnormalities in over 6000 cleavage-stage embryos. Reprod Biomed Online 14(5):628–634
Rubio C, Rodrigo L, Garcia-Pascual C, Peinado V, Campos-Galindo I, Garcia-Herrero S, Simón C (2019) Clinical application of embryo aneuploidy testing by next-generation sequencing. Biol Reprod 101(6):1083–1090
Baart EB, Martini E, Eijkemans MJ, Van Opstal D, Beckers NG, Verhoeff A, Macklon NS, Fauser BC (2007) Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod 22(4):980–988
Rubio C, Mercader A, Alama P, Lizan C, Rodrigo L, Labarta E, Melo M, Pellicer A, Remohí J (2010) Prospective cohort study in high responder oocyte donors using two hormonal stimulation protocols: Impact on embryo aneuploidy and development. Hum Reprod 25(9):2290–2297
Palmerola KL, Vitez SF, Amrane S, Fischer CP, Forman EJ (2019) Minimizing mosaicism: assessing the impact of fertilization method on rate of mosaicism after next-generation sequencing (NGS) preimplantation genetic testing for aneuploidy (PGT-A). J Assist Reprod Genet 36(1):153–157
Swain JE (2019) Controversies in ART: can the IVF laboratory influence preimplantation embryo aneuploidy? Reprod Biomed Online 39(4):599–607
Munné S, Alikani M, Ribustello L, Colls P, Martínez-Ortiz PA, McCulloh DH, Referring Physician Group (2017) Euploidy rates in donor egg cycles significantly differ between fertility centers. Hum Reprod 32(4):743–749
Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC (2013) Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60 000 cycles. Hum Reprod Update 19(5):433–457
Pardiñas ML, Nohales M, Laberta E, De Los Santos JM, Mercader A, Remohí J, Bosch E, De Los Santos MJ (2021) Elevated serum progesterone does not impact euploidy rates in PGT-A patients. J Assist Reprod Genet 38(7):1819–1826
Neves AR, Santos-Ribeiro S, García-Martínez S, Devesa M, Soares SR, García-Velasco JA, Garrido N, Polyzos NP (2021) The effect of late-follicular phase progesterone elevation on embryo ploidy and cumulative live birth rates. Reprod Biomed Online 43(6):1063–1069
Hernandez-Nieto C, Lee JA, Alkon-Meadows T, Luna-Rojas M, Mukherjee T, Copperman AB, Sandler B (2020) Late follicular phase progesterone elevation during ovarian stimulation is not associated with decreased implantation of chromosomally screened embryos in thaw cycles. Hum Reprod 35(8):1889–1899
Luciano A, Lodde V, Franciosi F, Ceciliani F, Peluso JJ (2010) Progesterone receptor membrane component 1 expression and putative function in bovine oocyte maturation, fertilization, and early embryonic development. Reproduction 140(5):663–672
Lodde V, Luciano AM, Garcia Barros R, Giovanardi G, Sivelli G, Franciosi F (2023) Review: the putative role of progesterone receptor membrane component 1 in bovine oocyte development and competence. Animal 17(Suppl 1):100783
Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Jenkins J, Pellicer A (2010) Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod 25(8):2092–2100
Garcia SPC, Pacheco FR, Mayo MM, Guerrero RR, Pareto SC (2024) Required number of blastocysts transferred, and oocytes retrieved to optimize live and cumulative live birth rates in the first complete cycle of IVF for autologous and donated oocytes. Arch Gynecol Obstet 310(5):2681–2690
Gardner DK, Schoolcraft WB (1999) Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol 11(3):307–311
Racca A, De Munck N, Santos-Ribeiro S, Drakopoulos P, Errazuriz J, Galvao A, Popovic-Todorovic B, Mackens S, De Vos M, Verheyen G, Tournaye H, Blockeel C (2020) Do we need to measure progesterone in oocyte donation cycles? A retrospective analysis evaluating cumulative live birth rates and embryo quality. Hum Reprod 35(1):167–174
Schoolcraft W, Sinton E, Schlenker T, Huynh D, Hamilton F, Meldrum DR (1991) Lower pregnancy rate with premature luteinization during pituitary suppression with leuprolide acetate. Fertil Steril 55(3):563–566
Xu B, Li Z, Zhang H, Jin L, Li Y, Ai J, Zhu G (2012) Serum progesterone level effects on the outcome of in vitro fertilization in patients with different ovarian response: an analysis of more than 10,000 cycles. Fertil Steril 97(6):1321–1327
Labarta E, Martínez-Conejero JA, Alamá P, Horcajadas JA, Pellicer A, Simón C, Bosch E (2011) Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: a functional genomics analysis. Hum Reprod 26(7):1813–1825
Van Vaerenbergh I, Fatemi HM, Blockeel C, Van Lommel L, In’t Veld P, Schuit F, Kolibianakis EM, Devroey P, Bourgain C (2011) Progesterone rise on hCG day in GnRH antagonist/rFSH stimulated cycles affects endometrial gene expression. Reprod Biomed Online 22(3):263–271
Horcajadas J, Riesewijk A, Polman J, Van Os R, Pellicer A, Mosselman S, Simón C (2005) Effect of controlled ovarian hyperstimulation in IVF on endometrial gene expression profiles. Mol Hum Reprod 11(3):195–205
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S (2010) Embryo cryopreservation rescues cycles with premature luteinization. Fertil Steril 93(2):636–641
Baldini D, Savoia M, Sciancalepore A, Malvasi A, Vizziello D, Beck R, Vizziello G (2018) High progesterone levels on the day of HCG administration do not affect the embryo quality and the reproductive outcomes of frozen embryo transfers. Clin Ter 169(3):e91–e95
Check JH, Wilson C, Choe JK, Amui J, Brasile D (2010) Evidence that high serum progesterone (P) levels on day of human chorionic gonadotropin (hCG) injection have no adverse effect on the embryo itself as determined by pregnancy outcome following embryo transfer using donated eggs. Clin Exp Obstet Gynecol 37(3):179–180
Melo MA, Meseguer M, Garrido N, Bosch E, Pellicer A, Remohí J (2006) The significance of premature luteinization in an oocyte-donation programme. Hum Reprod 21(6):1503–1507
Check JH, Hourani C, Choe JK, Callan C, Adelson HG (1994) Pregnancy rates in donors versus recipients according to the serum progesterone level at the time of human chorionic gonadotropin in a shared oocyte program. Fertil Steril 61(2):262–264
Huang B, Ren X, Wu L, Zhu L, Xu B, Li Y, Ai J, Jin L (2016) Elevated progesterone levels on the day of oocyte maturation may affect top quality embryo IVF cycles. PLoS ONE 11(1):e0145895
Vanni VS, Somigliana E, Reschini M, Pagliardini L, Marotta E, Faulisi S, Paffoni A, Vigano P, Vegetti W, Candiani M, Papaleo E (2017) Top quality blastocyst formation rates in relation to progesterone levels on the day of oocyte maturation in GnRH antagonist IVF/ICSI cycles. PLoS ONE 12(5):e0176482
Racca A, Santos-Ribeiro S, De Munck N, Mackens S, Drakopoulos P, Camus M, Verheyen G, Tournaye H, Blockeel C (2018) Impact of late follicular phase elevated serum progesterone on cumulative live birth rates: is there a deleterious effect on embryo quality? Hum Reprod 33(5):860–868
Lahoud R, Kwik M, Ryan J, Al-Jefout M, Foley J, Illingworth P (2012) Elevated progesterone in GnRH agonist down regulated in vitro fertilisation (IVFICSI) cycles reduces live birth rates but not embryo quality. Arch Gynecol Obstet 285(2):535–540
Ubaldi F, Smitz J, Wisanto A, Joris H, Schiettecatte J, Derde MP, Borkham E, Van Steirteghem A, Devroey P (1995) Oocyte and embryo quality as well as pregnancy rate in intracytoplasmic sperm injection are not affected by high follicular phase serum progesterone. Hum Reprod 10(12):3091–3096
Fanchin R, Righini C, Olivennes F, de Ziegler D, Selva J, Frydman R (1996) Premature progesterone elevation does not alter oocyte quality in in vitro fertilization. Fertil Steril 65(6):1178–1183
Silverberg KM, Martin M, Olive DL, Burns WN, Schenken RS (1994) Elevated serum progesterone levels on the day of human chorionic gonadotropin administration in in vitro fertilization cycles do not adversely affect embryo quality. Fertil Steril 61(3):508–513
Schneyer AL, Fujiwara T, Fox J, Welt CK, Adams J, Messerlian GM, Taylor AE (2000) Dynamic changes in the intrafollicular inhibin/activin/follistatin axis during human follicular development: relationship to circulating hormone concentrations. J Clin Endocrinol Metab 85(9):3319–3330
Shufaro Y, Sapir O, Oron G, Ben Haroush A, Garor R, Pinkas H, Shochat T, Fisch B (2015) Progesterone-to-follicle index is better correlated with in vitro fertilization cycle outcome than blood progesterone level. Fertil Steril 103(3):669–674
Aleksandrova N, Shubina E, Ekimov A, Kodyleva T, Mukosey I, Makarova N, Kulakova E, Levkov L, Trofimov D, Sukhikh G (2016) Comparison of the results of preimplantation genetic screening obtained by a-CGH and NGS methods from the same embryos. Gynecol Endocrinol 32(sup2):1–4
Acknowledgements
We would like to thank Creusa Maria Roveri Dal Bo for statistical analysis support. The authors wish to thank the clinicians, IVF embryologists and technicians involved in the Huntington’s oocyte donation program for their cooperation in the development of this study.
Funding
This research was partially supported by CNPq/Fapesp (304264/2021-0) and by Fundação de Amparo à pesquisa do estado de São Paulo (2018/24244-9).
Author information
Authors and Affiliations
Contributions
PLC contributed to the conception, design and writing of the manuscript. ARL performed data collection, statistical analysis and interpretation of data. She reviewed the intellectual content of the manuscript. APA, ELM and TSD participated in patient`s recruitment and care during medical treatment. BB performed IVF, embryo biopsy and data analysis. ELM, TSD, PAAM, ECB, PC and JMSJ contributed to the critical review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki and approved by the Ethical Committee of Clinical Research at Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (CAAE number 40900820.5.0000.0068).
Consent to participate
The study obtained exemption of the informed consent form as it was a retrospective research, using only the collection of data stored in medical records. There was no opposition from the subjects to use the data for the intended purpose.
Consent to publish
The authors are responsible for correctness of the statements provided in the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lopes Caldeira, P., Lorenzon, A.R., Chedraui, P. et al. The effect of progesterone level on day of trigger on embryo ploidy in egg donor’s cycles. Arch Gynecol Obstet 311, 765–774 (2025). https://doi.org/10.1007/s00404-025-07942-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-025-07942-7