Abstract
Purpose
We aimed to identify the correlation between morphological pronuclear (PN) status and the genetically determined ploidy configuration in preimplantation embryos.
Methods
A retrospective observational study was conducted on 1982 embryos displaying normal fertilization and 380 embryos showing an atypical PN pattern, tested for aneuploidies and ploidy status via preimplantation genetic testing (PGT) between May 2019 and May 2024. Ploidy prediction was performed using a validated targeted-NGS approach and a proprietary bioinformatic pipeline analyzing SNPs B-allele frequency information. Ploidy results were obtained in relation to the morphological PN pattern and further stratified by mode of PN observation, maternal age, and embryo quality parameters.
Results
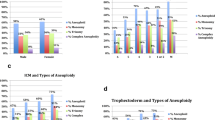
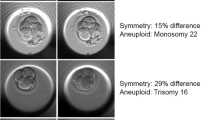
Abnormal ploidy results in 2PN-derived embryos were 1% (n = 20/1982): 0.8% showed triploidy and 0.2% haploidy. Ploidy results in relation to PN number in atypical fertilization were as follows: 0PN (n = 150/380) associated with 87.3% of diploidy, 8.7% of haploidy, and 4.0% of triploidy; 1PN-derived blastocysts (n = 73/153) were haploid in 47.7% of cases, 6.5% were triploid, and 45.7% diploid; 2.1PN (n = 23/280) and 3PN patterns (n = 54/280) predicted a triploid result in 34.8% and 74.1% of cases, respectively. PN observation with time-lapse increased ploidy status predictivity from 28.3% to 80.4% (p < 0.01) and reduced expected diploid rates to 19.6% (p < 0.01). Diploidy rate was higher for maternal age ≤ 35 years and for morphologically high-grade embryos.
Conclusion
Morphological PN check can be improved by incorporating ploidy analysis within the conventional PGT workflow. Euploid 2PN-derived embryos can be further selected removing haploids and triploids, and some atypical PN pattern can be better classified.






Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data Availability
The data underlying this article are available in the article and its online supplementary material.
References
De Los Santos MJ, Apter S, Coticchio G, Debrock S, Lundin K, Plancha CE, et al. Revised guidelines for good practice in IVF laboratories (2015). Hum Reprod. 2016;31:685–6.
Kemper JM, Liu Y, Afnan M, Mol BWJ, Morbeck DE. What happens to abnormally fertilized embryos? A scoping review Reprod Biomed Online. 2023;46:802–7. https://doi.org/10.1016/j.rbmo.2023.02.005.
Chen X, Shi S, Mao J, Zou L, Yu K. Developmental potential of abnormally fertilized oocytes and the associated clinical outcomes. Front Physiol. 2020;11:1–7.
Araki E, Itoi F, Honnma H, Asano Y, Oguri H, Nishikawa K. Correlation between the pronucleus size and the potential for human single pronucleus zygotes to develop into blastocysts: 1pn zygotes with large pronuclei can expect an embryo development to the blastocyst stage that is similar to the development of 2pn. J Assist Reprod Genet. 2018;35:817–23.
Bredbacka P, Capalbo A. Healthy live birth following embryo transfer of a blastocyst of tetrapronuclear (4PN) origin : a case report. Hum Reprod. 2023;38(9):1700–4. https://doi.org/10.1093/humrep/dead151.
Yao G, Xu J, Xin Z, Niu W, Shi S, Jin H, et al. Developmental potential of clinically discarded human embryos and associated chromosomal analysis. Sci Rep. 2016;6:1–9.
Destouni A, Dimitriadou E, Masset H, Debrock S, Melotte C, Van Den Bogaert K, et al. Genome-wide haplotyping embryos developing from 0PN and 1PN zygotes increases transferrable embryos in PGT-M. Hum Reprod. 2018;33:2302–11.
Capalbo A, Treff N, Cimadomo D, Tao X, Ferrero S, Vaiarelli A, et al. Abnormally fertilized oocytes can result in healthy live births: improved genetic technologies for preimplantation genetic testing can be used to rescue viable embryos in in vitro fertilization cycles. Fertil Steril. 2017;108:1007-1015.e3. https://doi.org/10.1016/j.fertnstert.2017.08.004.
Feenan K, Herbert M. Can, “abnormally” fertilized zygotes give rise to viable embryos? Hum Fertil. 2006;9:157–69.
Marin D, Zimmerman R, Tao X, Zhan Y, Scott RT, Treff NR. Validation of a targeted next generation sequencing-based comprehensive chromosome screening platform for detection of triploidy in human blastocysts. Reprod Biomed Online. 2018;36:388–95. https://doi.org/10.1016/j.rbmo.2017.12.015.
Kratka C, Vadapalli PS, Mendola R, Garrisi J, Xu J, Treff NR, et al. Accurate detection and frequency of abnormal ploidy in the human blastocyst. F S Sci. 2023;4:27–35. https://doi.org/10.1016/j.xfss.2023.02.003.
Joergensen MW, Labouriau R, Hindkjaer J, Stougaard M, Kolevraa S, Bolund L, et al. The parental origin correlates with the karyotype of human embryos developing from tripronuclear zygotes. Clin Exp Reprod Med. 2015;42:14–21.
Picchetta L, Figliuzzi M, Poli M, Zhan Y, Caroselli S, Tao X, et al. O-302 Triploid conceptions are predominantly caused by female meiosis II errors and their risk increases with advancing maternal age. Hum Reprod. 2023;38(Issue Supplement_1). https://doi.org/10.1093/humrep/dead093.366.
Mateo S, Parriego M, Boada M, Vidal F, Coroleu B, Veiga A. In vitro development and chromosome constitution of embryos derived from monopronucleated zygotes after intracytoplasmic sperm injection. Fertil Steril. 2013;99(3):897.
Grau N, Escrich L, Galiana Y, Meseguer M, García-Herrero S, Remohí J, et al. Morphokinetics as a predictor of self-correction to diploidy in tripronucleated intracytoplasmic sperm injection-derived human embryos. Fertil Steril. 2015;104:728–35.
Levy B, Sigurjonsson S, Pettersen B, Maisenbacher MK, Hall MP, Demko Z, et al. Genomic imbalance in products of conception: single-nucleotide polymorphism chromosomal microarray analysis. Obstet Gynecol. 2014;124:202–9.
Li M, Xue X, Zhang S, Li W, Zhao X, Ren W, et al. Effects of triploidy incidence on clinical outcomes for IVF-ET cycles in different ovarian stimulation protocols. Gynecol Endocrinol. 2015;31:769–73.
Caroselli S, Figliuzzi M, Picchetta L, Cogo F, Zambon P, Pergher I, et al. Improved clinical utility of preimplantation genetic testing through the integration of ploidy and common pathogenic microdeletions analyses. Hum Reprod. 2023;38:762–75.
García-Pascual CM, Navarro-Sánchez L, Navarro R, Martínez L, Jiménez J, Rodrigo L, et al. Optimized ngs approach for detection of aneu-ploidies and mosaicism in pgt-a and imbalances in pgt-sr. Genes (Basel). 2020;11:1–10.
Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4:1686.
Signorell A. DescTools: Tools for Descriptive Statistics. R package version 0.99.57. 2024. https://github.com/AndriSignorell/DescTools/, https://andrisignorell.github.io/DescTools/. Accessed 20 Mar 2024.
Frnk E. Harrell J. Regression modeling strategies with applications to linear models, logistic and ordinal regression, and survival analysis. Springer Ser Stat. 2016. https://doi.org/10.1007/978-3-319-19425-7
Rosenbusch BE. Mechanisms giving rise to triploid zygotes during assisted reproduction. Fertil Steril. 2008;90:49–55.
Brancati F, Mingarelli R, Dallapiccola B. Recurrent triploidy of maternal origin. Eur J Hum Genet. 2003;11:972–4.
Massalska D, Bijok J, Kucińska-Chahwan A, Zimowski JG, Ozdarska K, Panek G, et al. Triploid pregnancy–clinical implications. Clin Genet. 2021;100:368–75.
Jacobs BYPA, Angell RR, Buchanan IM, Hassold TJ, Matsuyama AM, Manuel B, et al. The origin of human triploids. Ann Hum Genet. 1978;42:49–57.
Staessen C, Van SAC. The chromosomal constitution of embryos developing from abnormally fertilized oocytes after intracytoplasmic sperm injection and conventional in-vitro fertilization. Hum Reprod. 1997;12:321–7.
Popescu F, Jaslow CR, Kutteh WH. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum Reprod. 2018;33:579–87.
Soler A, Morales C, Mademont-Soler I, Margarit E, Borrell A, Borobio V, et al. Overview of chromosome abnormalities in first trimester miscarriages: a series of 1,011 consecutive chorionic villi sample karyotypes. Cytogenet Genome Res. 2017;152:81–9.
Tong X, Jin J, Xue Y, Fang L, Zhu H, Jiang L, et al. Clinical outcomes of frozen–thawed blastocysts from zygotes with no or one pronucleus for in vitro fertilization and intracytoplasmic sperm injection cycles. Arch Gynecol Obstet. 2023;308:1015–22. https://doi.org/10.1007/s00404-023-07118-1.
Zhu J, Wang C, Cao Z, Luan K, Wu Y, Yin H. Developmental competence and neonatal outcomes of nonpronuclear zygotes following single vitrified-warmed blastocyst transfers using propensity score matching analysis. Arch Gynecol Obstet. 2024;309:295–304. https://doi.org/10.1007/s00404-023-07235-x.
Apter S, Ebner T, Freour T, Guns Y, Kovacic B, Le Clef N, et al. Good practice recommendations for the use of time-lapse technology. Hum Reprod Open. 2021;2020:1–26.
Kobayashi T, Ishikawa H, Ishii K, Sato A, Nakamura N, Saito Y, et al. Time-lapse monitoring of fertilized human oocytes focused on the incidence of 0PN embryos in conventional in vitro fertilization cycles. Sci Rep. 2021;11:1–7. https://doi.org/10.1038/s41598-021-98312-1.
Basile N, Nogales MDC, Bronet F, Florensa M, Riqueiros M, Rodrigo L, et al. Increasing the probability of selecting chromosomally normal embryos by time-lapse morphokinetics analysis. Fertil Steril. 2014;101(3):699-704.e1.
Bradley CK, Traversa MV, Hobson N, Gee AJ, McArthur SJ. Clinical use of monopronucleated zygotes following blastocyst culture and preimplantation genetic screening, including verification of biparental chromosome inheritance. Reprod Biomed Online. 2017;34:567–74. https://doi.org/10.1016/j.rbmo.2017.03.013.
Mateo S, Vidal F, Parriego M, Rodríguez I, Montalvo V, Veiga A, et al. Could monopronucleated ICSI zygotes be considered for transfer? Analysis through time-lapse monitoring and PGS. J Assist Reprod Genet. 2017;34:905–11.
Nagy ZP, Janssenswillen C, Janssens R, De Vos A, Staessen C, Van de Velde H, et al. Timing of oocyte activation, pronucleus formation and cleavage in humans after intracytoplasmic sperm injection (ICSI) with testicular spermatozoa and after ICSI or in-vitro fertilization on sibling oocytes with ejaculated spermatozoa. Hum Reprod. 1998;13:1606–12.
Staessen C, Janssenswillen C, Devroey P, Steirteghem ACV. Cytogenetic and morphological observations of single pronucleated human oocytes after in-vitro fertilization. Hum Reprod. 1993;8:221–3. Available from: https://pubmed.ncbi.nlm.nih.gov/8473423/. [cited 2023 Dec 21].
Van Der Heijden GW, Van Den Berg IM, Baart EB, Derijck AAHA, Martini E, De Boer P. Parental origin of chromatin in human monopronuclear zygotes revealed by asymmetric histone methylation patterns, differs between IVF and ICSI. Mol Reprod Dev [Internet]. 2009;76:101–8. Available from: https://pubmed.ncbi.nlm.nih.gov/18481364/. [cited 2023 Dec 21].
Azevedo AR, Pinho MJ, Silva J, Sá R, Thorsteinsdóttir S, Barros A, et al. Molecular cytogenetics of human single pronucleated zygotes. Reprod Sci [Internet]. 2014;21:1472–82. Available from: https://pubmed.ncbi.nlm.nih.gov/24717739/. [cited 2023 Dec 21].
Cimadomo D, Capalbo A, Scarica C, Sosa Fernandez L, Rienzi L, Ciriminna R, et al. When embryology meets genetics: the definition of developmentally incompetent preimplantation embryos (DIPE)—the consensus of two Italian scientific societies. J Assist Reprod Genet. 2021;38:319–31.
Xu J, Zhang M, Niu W, Yao G, Sun B, Bao X, et al. Genome-wide uniparental disomy screen in human discarded morphologically abnormal embryos. Sci Rep. 2015;5:1–10.
Grau N, Escrich L, Martín J, Rubio C, Pellicer A, Escribá MJ. Self-correction in tripronucleated human embryos. Fertil Steril. 2011;96:951–6. Available from: https://pubmed.ncbi.nlm.nih.gov/21851936/. [cited 2023 Dec 21].
Yalçınkaya E, Özay A, Ergin EG, Öztel Z, Özörnek H. Live birth after transfer of a tripronuclear embryo: an intracytoplasmic sperm injection as a combination of microarray and time-lapse technology. Turkish J Obstet Gynecol. 2016;13:95–8.
Canon C, Thurman A, Li A, Hernandez-Nieto C, Lee JA, Roth RM, et al. Assessing the clinical viability of micro 3 pronuclei zygotes. J Assist Reprod Genet. 2023. https://doi.org/10.1007/s10815-023-02830-y
Hattori H, Okuyama N, Ashikawa K, Sakuraba Y, Igarashi H, Kyono K. The utility of human two plus one small pronucleated zygotes (2.1PN) based on clinical outcomes and the focused ploidy analysis. J Assist Reprod Genet. 2024. https://doi.org/10.1007/s10815-024-03114-9
Wang J, Xiong S, Hao X, Gao Y, Xia F, Liao H, et al. Evaluating the developmental potential of 2.1PN-derived embryos and associated chromosomal analysis. J Assist Reprod Genet. 2024. https://doi.org/10.1007/s10815-024-03113-w
McFadden DE, Langlois S. Parental and meiotic origin of triploidy in the embryonic and fetal periods. Clin Genet. 2000;58:192–200.
Ezoe K, Takahashi T, Shimazaki K, Miki T, Tanimura Y, Amagai A, et al. Human 1PN and 3PN zygotes recapitulate all morphokinetic events of normal fertilization but reveal novel developmental errors. Hum Reprod. 2022;37:2307–19.
Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29:1173–81.
Fragouli E, Alfarawati S, Spath K, Wells D. Morphological and cytogenetic assessment of cleavage and blastocyst stage embryos. Mol Hum Reprod. 2014;20:117–26.
McCoy RC, Summers MC, McCollin A, Ottolini CS, Ahuja K, Handyside AH. Meiotic and mitotic aneuploidies drive arrest of in vitro fertilized human preimplantation embryos. Genome Med. 2023;15:77. https://doi.org/10.1186/s13073-023-01231-1.
Destouni A, Esteki MZ, Catteeuw M, Tšuiko O, Dimitriadou E, Smits K, et al. Zygotes segregate entire parental genomes in distinct blastomere lineages causing cleavage-stage chimerism and mixoploidy. Genome Res. 2016;26:567–78.
Carson JC, Hoffner L, Conlin L, Parks WT, Fisher RA, Spinner N, et al. Diploid/triploid mixoploidy: a consequence of asymmetric zygotic segregation of parental genomes. Am J Med Genet Part A. 2018;176:2720–32.
De Coster T, Masset H, Tšuiko O, Catteeuw M, Zhao Y, Dierckxsens N, et al. Parental genomes segregate into distinct blastomeres during multipolar zygotic divisions leading to mixoploid and chimeric blastocysts. Genome Biol. 2022;23:1–29. https://doi.org/10.1186/s13059-022-02763-2.
Acknowledgements
The authors acknowledge Igenomix staff from the R&D, laboratory and diagnosis departments of Italy, Japan, USA, Brazil, and Spain.
Funding
This work was supported by Igenomix.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors are employees of Igenomix, a company providing reproductive genetic services, part of Vitrolife Group.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Girardi, L., Patassini, C., Miravet Valenciano, J. et al. Incidence of haploidy and triploidy in trophectoderm biopsies of blastocysts derived from normally and abnormally fertilized oocytes. J Assist Reprod Genet 41, 3357–3370 (2024). https://doi.org/10.1007/s10815-024-03278-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-024-03278-4